Anion Exchange Membranes
Anion Exchange Membranes
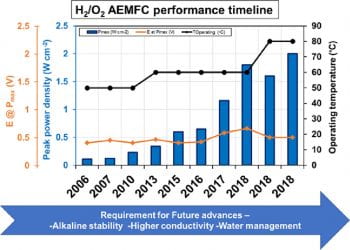
Hydroxide-ion-conducting anion exchange membranes (AEMs) facilitate the use of inexpensive non-PGM electrocatalysts in fuel cells but have traditionally from low conductivity and poor durability. Our group has established the relationship between cation basicity (pKa) and ionic conductivity in AEMs and has pioneered the use of 2D NMR spectroscopy and other spectroscopic tools to identify AEM cation and backbone degradation mechanisms/pathways. These mechanistic insights have prompted new synthetic directions that have yielded more robust AEMs for use in fuel cells, electrolyzers, and redox flow batteries for grid-scale electric energy storage.
Featured Papers:
“Detection of reactive oxygen species in anion exchange membrane fuel cells using in situ fluorescence spectroscopy”, Y. Zhang, J. Parrondo, S. Sankarasubramanian, & V. Ramani, ChemSusChem, 10(15), pp. 3056-3062, (2017).
“Best Practices for Investigating Anion Exchange Membrane Suitability for Alkaline Electrochemical Devices: Case Study Using Quaternary Ammonium Poly(2,6-dimethyl 1,4-phenylene)oxide Anion Exchange Membranes”, C. G. Arges, L. Wang, J. Parrondo, & V. Raman, J. Electrochem. Soc., 160(11), pp. F1258-F1274, (2013).
Bipolar Interfaces For Energy Conversion

Bipolar Interfaces For Energy Conversion
We have designed and demonstrated a microscale bipolar interface that enables exceptionally sharp pH gradients (ca. 1 pH unit/nm) at the electrolyte/electrode interface of an electrochemical cell. We have demonstrated that this approach enables hitherto unachievable high-power electrochemical cell operation using unique redox chemistries. We use this design in liquid-liquid fuel cells, regenerative fuel cells, and electrolyzers (water, CO2 etc.).
Featured Paper:
“Efficient pH-gradient-enabled microscale bipolar interfaces in direct borohydride fuel cells”, Z. Wang, J. Parrondo, C. He, S. Sankarasubramanian, & V. Ramani, Nature Energy, 4(4), pp. 281-289, (2017).
Proton Exchange Membranes

Proton Exchange Membranes
We have significant expertise with the manufacture of numerous stand-alone, reinforced, and composite/hybrid proton conducting membranes for electrochemical energy conversion and storage as well as separation applications. Additionally, we have developed advanced diagnostic methods to explore oxidative degradation in these membranes, and have invented mitigation strategies that obviate oxidative degradation through reactive oxygen species capture.
Featured Papers:
“Structurally-tuned nitrogen-doped cerium oxide exhibits exceptional regenerative free radical scavenging activity”, V. Prabhakaran & V. Ramani, J. Electrochem. Soc., 161(1), pp. F1-F9, (2014).
“Investigation of PEM chemical degradation and degradation mitigation using in-situ fluorescence spectroscopy”, V. Prabhakaran, C. Arges, V. Ramani, Proceedings of the National Academy of Sciences, USA, 109(4), pp. 1029-1034, (2012).