News prior to 2019 archived
Noyonika Joins the Lab
By Erika Brown – November 4, 2024

The Millman Lab welcomes Noyonika Mukherjee to the lab as a postdoctoral researcher. Noyonika, born and raised in Kolkata, West Bengal, India, earned her BS and MS in Microbiology from the University of Calcutta. In 2019, she began her doctoral studies in Biochemistry and Molecular Biology at the Indiana University School of Medicine, Indianapolis, IN, USA, under the guidance of Dr. Andrew T. Templin. Noyonika’s doctoral research was focused on uncovering lesser-known mechanisms of beta-cell cytotoxicity, particularly examining how receptor-interacting protein kinases contribute to islet inflammation and beta-cell death in diabetes pathogenesis. In Fall 2024, Noyonika was awarded the Rita Levi-Montalcini postdoctoral fellowship from Washington University in St. Louis to pursue research in regenerative medicine. She subsequently joined Dr. Jeffrey R. Millman’s lab as a postdoctoral research fellow. Building on her expertise in beta-cell cytotoxic responses, Noyonika aims to integrate the Millman Lab’s advancements in SC-derived beta cell development into her work. Her goal is to create iPSC-derived exocrine and endocrine organoid systems that will deepen our understanding of how environmental factors impact type 1 diabetes. When not in the lab, Noyonika can be found in the kitchen trying out new baking recipes!
Marlie Awarded Winston Fellowship
By Erika Brown – October 25, 2024

Marlie Maestas was one of two 2024 Winston Fellowship awardees. The David and Deborah Winston Fellowship in Diabetes Research was funded in 2012 to support graduate students conducting diabetes and diabetes-related research in the labs of Diabetes Research Center (DRC) members. The fellowship is now awarded annually from the Division of Endocrinology, Metabolism & Lipid Research to outstanding graduate students who show great promise in diabetes research. It provides monetary support for the recipient(s) research and research-related activities. Congratulations, Marlie!
$2.75 million to support type 1 diabetes research
By Constance Gibbs – October 17, 2024

The Division of Endocrinology, Metabolism & Lipid Research in the Department of Medicine has received a $2.75 million gift through the Anita Palmer Corbin Trust to establish the Anita Palmer Corbin Diabetes Research Endowed Fund. The fund augments a spendable fund to support diabetes research in the division that was previously created by Corbin, who died in 2023.
Diagnosed with Type 1 diabetes at age 10, Corbin learned early in life to meet challenges head-on. She enjoyed a more than 20-year career at St. Louis-based Ralston Purina Co., starting as a senior accountant in 1979. After holding several positions of increasing responsibility, she was named vice president and controller in 1994, becoming the first female officer in the company. Her husband, Daniel E. Corbin Jr., said that while his wife championed many causes throughout her life, diabetes research was among her top priorities.
Annual payout from the endowed fund will be used to support Type 1 diabetes research conducted by Jeffrey Millman, PhD, professor of medicine. Millman successfully converted human stem cells into insulin-producing beta cells that were able to rapidly cure diabetes in mice. He has been honing the process to improve the survival and function of insulin-producing beta cells transplanted into patients.
“We are deeply grateful to Anita for her generous support of diabetes research. Discoveries from the Millman laboratory could help revolutionize treatment for individuals with Type 1 diabetes. Cellular replacement therapy offers patients a chance to have a healthier life and a more hopeful future.”
Clay F. Semenkovich, MD, chief of the Division of Endocrinology, Metabolism & Lipid Research and the Irene E. and Michael M. Karl Professor
Meet the Researcher: Danny Veronese-Paniagua
By Erika Brown – October 1, 2024
Daniel Veronese-Paniagua, a sixth-year graduate student in the Millman Lab, recently published his first first-authorship as a preprint in bioRxiv. The study, titled Coxsackievirus B infection invokes unique cell-type specific responses in primary human pancreatic islets, sheds new light on the intricate mechanisms through which Coxsackievirus B3 (CVB) may trigger type 1 diabetes (T1D). The study was conducted by Veronese-Paniagua, Dr. Jeffrey Millman, Dr. Hubert Tse of the University of Kansas Medical Center, and their colleagues at Washington University School of Medicine and the University of Kansas Medical Center. It utilized single-cell RNA sequencing to uncover distinct responses within pancreatic islet cell types—specifically β, α, and ductal cells—following CVB infection.
CVB has long been suspected to play a role in the onset of T1D, an autoimmune condition in which the immune system attacks insulin-producing β cells in the pancreas. Veronese-Paniagua’s study provides crucial insights into how this virus induces transcriptional changes that disrupt cellular functions vital for maintaining glucose homeostasis. This preprint publication marks a significant milestone in advancing the understanding of the link between viral infections and the development of T1D, offering new avenues for clinical research and treatment development. It underscores the complex interplay between viral infections, cellular responses, and autoimmune diseases like T1D. As research continues to unravel these connections, there is hope for future treatments that could preserve pancreatic function and improve outcomes for individuals at risk of developing diabetes.
Veronese-Paniagua grew up in an underserved community and attended a high school with a limited science department. “When I started college, I knew I liked science, but my only concepts of science were engineering, medicine, and teaching. I knew I didn’t want to teach, I knew I didn’t want to be an engineer, so I said to myself, ‘Okay, my next thing is to go into medicine.’ Thankfully, within my first year of college, I discovered the world of research, and I fell in love with it.” He finds it “addicting” to discover new things in research. “[It’s addicting] to have a question and to find an answer that may fit your expectations, but it has more to it, it’s more complex than you thought it was. You expect the complexity, but sometimes the level of complexity is beyond your imagination.”
After earning his Bachelor of Arts in Biology from Cornell, he worked as a research technician at Washington University for two years, studying colorectal cancer in the Blair Madison Lab. This experience sparked his interest in stem cell biology. While Veronese-Paniagua initially wanted to move on from Washington University after his time in the Madison Lab, he later realized and appreciated the school’s strong student support and diversity, which made him feel more comfortable than other places he interviewed at. Veronese-Paniagua joined the Millman Lab in 2020 as a student in the Developmental, Regenerative, and Stem Cell Biology Program. He was attracted to the lab’s working dynamic as well as the translational research. “You can see the fruit from your seed grow during your own lifespan. We see that with Jeff’s (Millman) 2014 paper already in clinical trials less than 10 years later,” he said.
After graduation, Veronese-Paniagua plans to work in the private sector. He’s interested in working for a pharmaceutical or biotech company to develop tools that can reach patients within his lifetime. He says that he enjoys the concept of seeing the effects of his work. Further down the line, he envisions himself working in an administrative capacity, making scientific decisions within a company.
Veronese-Paniagua understands the importance of maintaining a healthy work-life balance and emphasizes the value of having hobbies and a social circle outside of science. One of his passions outside of the lab is practicing Brazilian Jiu-Jitsu. He has been practicing Jiu-Jitsu at Watson Martial Arts in St. Louis for seven years and says he loves the sport because of the way it slows down the world. “It makes you think a lot about what the movements you have to do against your opponent are. Although to a spectator, it may look fast paced, when you’re on the mats it kind of slows you down, slows your thinking down, and makes you forget about the world because all you’re thinking about is the moves you want to do. It’s a really easy way for me to forget about science,” he said. He also appreciates how the sport tests both his physical and mental abilities. “It’s one of those communities where you can continuously learn from people regardless of their background. I’ve gotten my butt handed to me by people that are in their sixties. And vice versa. I’ve beat people 100 pounds heavier than me. And you can’t do that in other sports for the most part because strength, power, height, age – all those factors come in. But Jiu-Jitsu’s one of those things where you have a little bit more control, and I really like that.”
Additionally, he is dedicated to supporting the younger scientific community, especially in underserved communities. He volunteers with WashU’s Teach Kids Science, a program that brings graduate students to schools and communities in St. Louis, particularly in underserved areas of the city, to provide talks and scientific demonstrations for students in grades K-12. “I think it’s more important when they learn that from people that look like them and showing them that we were in your shoes at some point in life and now we’re here, and it can be you too,” Veronese-Paniagua said. He finds joy in tutoring young scientists and helping them understand the opportunities available to them. “It’s really cool because I’ve spoken to some kids that really like science, but kind of like me when I was in high school, they don’t know what they can do with science.”
Read more about Veronese-Paniagua’s study in bioRxiv here.
Millman Lab Walks for Breakthrough T1D
By Erika Brown – September 30, 2024
Several members of the Millman Lab, along with their families and furry friends, came together for the Breakthrough T1D Walk on Sunday, September 29. This one-mile walk featured additional activities and is designed to raise funds for diabetes research while providing a supportive space for those living with Type 1 Diabetes, their families, and friends. Following a week of rain, most lab attendees sported muddy paw prints on their shirts, evidence of the day’s fun. It was a wonderful opportunity to unite and show our support for the Breakthrough T1D community!
Matt Interviewed by 10x Genomics
By Erika Brown – September 25, 2024

Dr. Matt Ishahak was recently interviewed by 10x Genomics was recently interviewed by 10x Genomics about his groundbreaking work on engineered glioblastoma brain organoids (eGBOs). This innovative model aims to expedite therapeutic development for glioblastoma (GBM), a particularly aggressive brain cancer with a five-year survival rate of less than 5%.
In the interview, Dr. Ishahak emphasized the advantages of using eGBOs over traditional cancer models. “GBM tumors are super complex,” he explained. “Patients often don’t seek treatment until the cancer is very advanced, meaning their tumors have a high mutational load from the start. Our eGBOs enable insights into early tumor initiation mechanisms. By introducing just a few mutations early on, we can see how that develops into these complex cancer phenotypes often found in patients.”
Check out the whole interview here.
Alice Joins the Lab
By Erika Brown – September 25, 2024

The Millman Lab welcomes Alice Smagorinsky to the lab as an undergraduate researcher. Alice is a freshman at WashU from Northbrook, Illinois. She plans to major in biology on the molecular biology and biochemistry track and minor in computer science. Alice has always been fascinated with biology, and her Type 1 diabetes diagnosis in 2017 furthered her interest in a research career. In her free time, Alice loves to hike, work out, watch stand-up comedy, and spend time with her friends. Welcome, Alice!
Mira Gains Citizenship
By Erika Brown – September 20, 2024

Millman Lab member Mira Shunkarova became a US citizen during a ceremony in Belleville, Illinois last week. Mira immigrated from Kazakhstan with her son and husband in 2021, living in the DC area before moving to her husband’s hometown of O’Fallon a year later. Her husband and in-laws were in attendance at the event. Congratulations, Mira!
New Publication
By Erika Brown – September 4, 2024

Dr. Millman and his colleagues published a comprehensive review article titled “Harnessing Cellular Therapeutics for Type 1 Diabetes Mellitus: Progress, Challenges, and the Road Ahead” in Nature Reviews Endocrinology. This article offers an in-depth analysis of the advancements in cell replacement therapies for treating Type 1 diabetes mellitus (T1DM). The review begins by discussing the current state of pancreatic islet transplantation as a potential cure for T1DM, noting the significant challenges posed by donor scarcity, immune rejection, and the requirement for lifelong immunosuppression.
A major focus of the article is the generation of replenishable β cells from human pluripotent stem cells (hPSCs) as an alternative to donor islets. While these stem cell-derived islets hold significant promise, they face ongoing challenges related to functionality, safety, and scalability. The authors discuss various strategies, including microencapsulation and macroencapsulation, designed to protect transplanted cells from immune rejection without the need for systemic immunosuppression.
The review emphasizes the critical importance of preserving cell viability, engraftment, and functionality through innovative delivery methods. Emerging approaches to localized immunomodulation and reducing the immunogenicity of transplanted β cells are explored, with the goal of achieving long-term immune tolerance and minimizing the need for chronic systemic immunosuppression.
The article also underscores the essential role of animal models in advancing T1DM research, particularly in evaluating cell transplantation and immune modulation strategies. Additionally, the review outlines the challenges and future directions for translating these therapies into clinical practice, emphasizing the need for scalable, safe, and effective products that align with real-world healthcare dynamics.
Overall, this article serves as a roadmap for the development of β cell replacement therapies, advocating for integrated approaches to overcome the technical, regulatory, and clinical challenges that lie ahead.
Check out the article here.
Promotion Celebration
By Erika Brown – August 22, 2024
The Division of Endocrinology, Metabolism & Lipid Research hosted a celebratory party on Thursday to honor the promotion of four esteemed faculty members to full professors. Dr. Jeffrey Millman, Dr. Irfan Lodhi, Dr. Maria Remedi, and Dr. Julie Silverstein each received their full professorships and have each made substantial contributions to the field of diabetes research.
Dr. Clay Semenkovich, division chief, addressed attendees, acknowledging the newly promoted professors’ contributions to diabetes research within their careers at WashU.
The event was catered by Across the Board 314, a locally, queer-, and woman-owned charcuterie board company known for its commitment to sustainability.
Congratulations to Drs. Millman, Lodhi, Remedi, and Silverstein!
New Publication in Stem Cells Translational Medicine
By Erika Brown – August 21, 2024
Marlie Maestas, Maggie Bui, and Dr. Millman published a review article in Stem Cells Translational Medicine titled “Recent progress in modeling and treating diabetes using stem cell-derived islets.” The article highlights multiple diabetes-associated diseases that use stem cell-derived islets (SC-islets) for disease modeling and the transplantation of stem cell-derived islets for functional cell therapy. By employing genetic modifications and chemical compounds, researchers can now model diabetes more effectively in vitro, providing valuable insights into disease mechanisms. In addition to detailing these modeling techniques, the review explores strategies for improving SC-islet therapies through immune modulation. By enhancing the body’s acceptance of transplanted islets, these methods aim to boost the efficacy of treatments and offer new hope for patients with diabetes.
Read the article here for more.
Millman Lab Members Place in Local Run
By Erika Brown – August 13, 2024

Left to right: Tai Le, Allison Kelley with Kipper, Ed Sanchez Castro, Marlie Maestas, and Matt Ishahak with Leary
Members of the Millman Lab took a break from their scientific work and participated in the Drink MO Beer Run on Saturday, August 10 at Creve Coeur Park. Participants had the option to run either one mile, a 5K, or a 10K. The event ended with a festival that included beer from local microbreweries, food, and live music.
Some of the lab members took home awards for their placements in the race. In the 10K race, Marlie Maestas secured second place in the female age 20-29 category, and Ed Sanchez Castro claimed the top spot in the male age 20-29 category. This team’s impressive results were further highlighted by Leary, Matt Ishahak’s dog, who placed first in the 5K Doggo group.
Congratulations to all!
New Preprint From the Millman Lab
By Erika Brown – August 5, 2024

In a recent preprint on bioRxiv from the Millman lab titled “Modeling glioblastoma tumor progression via CRISPR-engineered brain organoids,” Dr. Matthew Ishahak and colleagues explore the use of engineered glioblastoma organoids (eGBOs) to investigate tumor progression in glioblastoma (GBM), an aggressive form of brain cancer. GBM is highly resistant to therapy and exhibits significant intratumoral heterogeneity. Traditional models like genetically modified mice and patient-derived cancer spheroids have limitations in studying early tumor progression and often lack human relevance. In contrast, human pluripotent stem cell-derived brain organoids offer a promising in vitro system to model human neural development and diseases, including cerebral tumors.
The primary objective of this study was to develop eGBOs with subtype-specific oncogenic mutations to investigate the transcriptional regulation and progression of tumors. Researchers used CRISPR-Cas9 to introduce specific GBM-associated mutations into human pluripotent stem cells (hPSCs). To assess the transcriptional and spatial organization of eGBOs, they employed single-cell RNA sequencing (scRNAseq) and spatial transcriptomic analyses. Finally, eGBOs were transplanted into immunodeficient mice to evaluate their ability to form tumors and recapitulate the characteristics of human GBM.
The key findings from the study are that eGBOs with GBM-specific mutations exhibited altered gene regulatory networks, leading to changes in cellular composition and spatial organization. When transplanted into mice, eGBOs formed tumors that mirrored the transcriptional and spatial landscape of human GBM samples. Single-cell trajectory analysis identified dynamic gene expression changes associated with developmental cell states underlying tumor progression. Moreover, eGBO-derived tumors displayed subtype-specific characteristics, demonstrating their utility as models for GBM tumorigenesis.
The study validates the use of engineered cancer organoid models for GBM and underscores their potential in preclinical drug development. eGBOs provide a robust platform for studying GBM tumor progression and investigating new therapeutic approaches. This model system helps bridge the gap between preclinical findings and clinical applications, potentially leading to the development of more effective treatments for GBM. By offering a more human-relevant model for understanding GBM progression, eGBOs hold promise for advancing the research and treatment of this formidable disease.
About the Researchers
This research was conducted by a team of scientists, including Dr. Matthew Ishahak, Dr. Jeffrey Millman, Dr. Albert Kim, and their colleagues at Washington University School of Medicine.
For further details on the study, the preprint publication can be accessed here.
Meet the Researcher: Marlie Maestas
By Erika Brown – August 2, 2024

Marlie Maestas, a fifth-year graduate student in the Millman Lab, recently published her first first-authorship in Nature Communications. The study, titled Identification of unique cell type responses in pancreatic islets to stress, detailed the responses of different pancreatic islet cell types to endoplasmic reticulum and inflammatory stress. Maestas and her colleagues used single-cell RNA sequencing to detect stress’s impact on gene expression in each cell type and demonstrated that each type responds differently. The study indexed these responses and identified beta-cell-specific responses. Additionally, Maestas, Millman Lab and Urano Lab used their findings to identify the candidate gene CIB1 as a potential regulator of beta cell function.
“This study was designed to utilize multiplexed sequencing to understand the effects of different types of cell stress and reduce the variability due to multiple sequencing runs,” Maestas said. “Previous studies have investigated the compounds we have tested here; however, we noticed that these studies did not investigate cell-type-specific responses, leading us to design this study.”
The findings provide a detailed map of how pancreatic cells react to stress, offering insights that could lead to new therapeutic strategies. By targeting specific stress pathways, it may be possible to protect β-cells from damage and improve the overall health of pancreatic islets in diabetic patients.
Maestas received her Bachelor of Science in Biology from New Mexico State University and says she picked Washington University for graduate school because of the community. “When I came for my interview, I really liked all of the people and I felt like I would really be a part of a community,” she said. In 2021, Maestas joined the Millman Lab as a doctoral student in developmental, regenerative, stem cell biology. Although she initially joined the lab because of the people and the environment, she really enjoys the translational aspects of the lab and how much of an impact the studies have on diabetes research. Having family and friends who have diabetes has made her even more interested in the research.
After graduate school, Maestas plans to work in the biotechnology industry, designing new products or medicine to continue helping patients. She talked about shadowing a physician assistant during her undergraduate studies. “Every day she gave patients new medicines to try to see if they would help. While shadowing, I realized I wanted to be the person involved in understanding and creating new medicines for people.”
When she’s not working in the lab, Maestas likes to travel, hang out with her friends, and get lost in a good book. Her favorite destination so far is the Philippines, where she loved the people, food, and landscapes.
Read more about Maestas’s study in Nature Communications here.
James Joins the Lab
By Erika Brown – July 29, 2024

The Millman Lab welcomes James Lu to the lab as a post-baccalaureate scholar in the Division of Cellular and Biomedical Research and Medicine (DCBRM). James, a recent graduate of the University of Oklahoma, holds both a B.S. in Biological Sciences and a B.A. in Spanish Linguistics and Literature, having completed his studies in May 2024. James is from Tulsa, OK, and is looking forward to learning more in the lab to further his interests in advancing treatment and management strategies for chronic illnesses, particularly diabetes and obesity. Outside of lab, James enjoys pickleball, exploring local cuisine, and experimenting with cooking. Welcome, James!
New Preprint From the Millman Lab
By Erika Brown – July 24, 2024

In a recent preprint from the Millman Lab titled “Coxsackievirus B infection invokes unique cell-type specific responses in primary human pancreatic islets,” Daniel Veronese-Paniagua and colleagues have shed new light on the intricate mechanisms through which Coxsackievirus B3 (CVB) may trigger type 1 diabetes (T1D). Published as a preprint in bioRxiv, the study utilized single-cell RNA sequencing to uncover distinct responses within pancreatic islet cell types—specifically β, α, and ductal cells—following CVB infection.
CVB has long been suspected to play a role in the onset of T1D, an autoimmune condition where the immune system attacks insulin-producing β cells in the pancreas. Veronese-Paniagua’s study provides crucial insights into how this virus induces transcriptional changes that disrupt cellular functions crucial for maintaining glucose homeostasis.
Key Findings:
- Cell-Specific Responses: The research identified unique transcriptional responses in different cell types within pancreatic islets. β cells, critical for insulin production, showed significant changes linked to mitochondrial dysfunction, immune responses, and inflammation pathways upon CVB infection.
- Impact on Mitochondrial Function: CVB infection was found to disrupt oxidative phosphorylation pathways in β cells, suggesting a direct mechanism through which viral infection impairs energy production and cellular function.
- Role of Long Non-Coding RNA: The study highlighted the upregulation of MIR7-3HG, a long non-coding RNA, in β cells with high viral loads. Knockdown experiments demonstrated that reducing MIR7-3HG levels could potentially mitigate viral protein expression and apoptosis in β cells, pointing to a novel therapeutic target.
- Implications for Therapy: These findings offer promising avenues for therapeutic intervention aimed at protecting β cells from viral-induced damage and subsequent autoimmune attacks in individuals predisposed to T1D.
Veronese-Paniagua emphasized that understanding the molecular pathways activated by CVB in pancreatic islet cells is crucial for developing targeted therapies that could potentially prevent or mitigate the progression of T1D in susceptible individuals.
This study underscores the complex interplay between viral infections, cellular responses, and autoimmune diseases like T1D. As research continues to unravel these connections, there is hope for future treatments that could preserve pancreatic function and improve outcomes for individuals at risk of developing diabetes.
Implications for Diabetes Treatment
This preprint publication marks a significant milestone in advancing our understanding of the link between viral infections and the development of T1D, offering new avenues for clinical research and treatment development.
About the Researchers
This research was conducted by a team of scientists including Daniel Veronese-Paniagua, Dr. Jeffrey Millman, Dr. Hubert Tse of the University of Kansas Medical Center, and their colleagues at Washington University School of Medicine and University of Kansas Medical Center.
For further details on the study, the preprint publication can be accessed here.
Contributions to the 84th Scientific Sessions
By Courtney Casteel – July 24, 2024
Many members of the Division of Endocrinology, Metabolism and Lipid Research participated at the American Diabetes Association‘s 84th Scientific Sessions, which took place in Orlando, Florida from June 21-24. Those members include Esmeralda Castelblanco Echavarria, PhD; Kai Jones, MD; Jeffrey R. Millman, PhD; Max C. Petersen, MD, PhD; R. Mei Zhang, MD; and Wei Zhang, PhD.
As the world’s largest meeting on diabetes, the sessions have welcomed colleagues and experts from over 115 countries to promote collaboration and scientific advancement in the field. The sessions encompassed eight theme areas that allowed attendees to learn about the latest scientific findings, groundbreaking research presentations, and to experience the exhibit and poster halls with the intention of accelerating the progression toward diabetes prevention and finding a cure.
Division Participants

Esmeralda Castelblanco Echavarria, PhD
Poster Presentation
Theme Area: Clinical and Preclinical Pancreas Immunology and Islet function
Session: Beta Cell Signal Transduction
Restoring beta-cell Mass and Identity in KATP-Induced Neonatal Diabetes – The Synergy of Autophagy and Intermittent Fasting

Kai Jones, MD
Oral Presentation
Theme Area: Clinical Diabetes/Therapeutics
Session: “New Technology—What’s New?”
Breath Acetone Correlates with Capillary Beta Hydroxybutyrate in Type 1 Diabetes

Jeffrey R. Millman, PhD
Oral Presentation
Theme Area: Immunology/Beta-Cell Replacement
Session: The “Barrier Reef”—Overcoming Hurdles to Beta-Cell Replacement Therapies Overcoming Islet Scarcity through In Vitro Differentiation of Human Pluripotent Stem Cells

Dr. Millman also participated in an expert panel discussing the heterogeneity of type 1 diabetes (T1D), covering topics such as beta cell pathology, T1D prevention, and insulin replacement. He was one of the experts selected to later present a summary of the panel discussion. In addition, Dr. Millman joined a public panel on the subject and will be co-authoring a review article with his co-panelists to further disseminate their findings.

Max C. Petersen, MD, PhD
Poster Presentation
Theme Area: Insulin Action/Molecular Metabolism
Session: Metabolite Transport and Molecular Metabolism
Effect of Dapagliflozin on Blood and Breath Ketones During Supervised Insulin Withdrawal in Adults with Type 1 Diabetes
Oral Presentation
Theme Area: Obesity/Integrated Physiology
Session: Risk Factors Reimagined—Beyond Obesity in Metabolic Risk
Plasma Lipidome in People with Insulin-Sensitive and Insulin-Resistant Obesity

R. Mei Zhang, MD
Poster Presentation
Theme Area: Behavioral Medicine, Clinical Nutrition, Education, and Exercise
Session: Type 2 Diabetes and Exercise—Where Do We Stand?
Acute Hyperglycemia Induces Human Podocyte Apoptosis by Increasing Monocyte Release of TNF-a Through ER Stress/ROS Activation of ADAM17/iRhom2 Expression

Wei Zhang, PhD
Oral Presentation
Theme Area: Insulin Action/Molecular Metabolism
Session: Novel Regulators of Insulin Action and Insulin Sensitivity
Depalmitoylation Inhibition Improves Glucose Tolerance by Promoting Endothelial Insulin Transcytosis
Additionally, Dr. Zhang was named a recipient of the Young Investigator Award and received one of the top scores within the program.
Millman Lab Participates in Neighborhood Cleanup
By Erika Brown – July 13, 2024

Members of the Millman Lab and their families rolled up their sleeves and joined forces with local residents for a neighborhood cleanup in Normandy, MO on Saturday, July 13. This event was organized as part of earthday365‘s Environmental Justice Days of Action and co-hosted by Metropolitan Congregations United (MCU).
Environmental Justice Days of Action are community service focused days aimed at addressing food apartheid, litter, and illegal dumping typically in poor or marginalized communities. This cleanup focused on addressing litter and illegal dumping in the underserved community of Normandy, a city in St. Louis County.
The event concluded with a group discussion about how policy and education can help pave the way for a cleaner, healthier Earth. With continued efforts like these, organizers hope to inspire ongoing environmental stewardship and community involvement beyond Environmental Justice Days of Action.
New Publication in Nature Communications
By Erika Brown – July 3, 2024

Recent research published in Nature Communications sheds light on how different cell types in the pancreas respond to stress, revealing potential new targets for diabetes treatment. The study, led by Marlie Maestas of the Millman laboratory at Washington University School of Medicine, focuses on the pancreatic islets, which contain cells crucial for regulating blood sugar.
Diabetes, a chronic condition affecting millions worldwide, involves the malfunction or death of pancreatic β-cells, which produce insulin. Insulin is essential for maintaining normal blood sugar levels. The study investigates how these cells, along with other cell types in the pancreatic islets, respond to two major stressors: endoplasmic reticulum (ER) stress and inflammatory stress.
Key Findings:
- Cell-Specific Stress Responses: The researchers used advanced single-cell RNA sequencing to analyze how different cell types in the pancreas react to stress. They found that β-cells, α-cells, and ductal cells have the most significant responses to stress. β-cells, responsible for insulin production, showed extensive changes in gene expression when exposed to stress.
- ER Stress and Inflammatory Stress: ER stress occurs when the ER, a cell structure involved in protein folding, becomes overwhelmed with misfolded proteins. This can happen due to various factors, including high blood sugar and inflammation. The study identified that β-cells under ER stress had increased expression of genes related to cell death, while α-cells (which produce glucagon) showed increased expression of survival genes.
- Potential Therapeutic Targets: One of the significant findings was the role of a gene called CIB1, which was upregulated in stressed β-cells. When the researchers modified stem cell-derived islets to overexpress or knock down CIB1, they observed changes in insulin secretion and cell survival. This suggests that CIB1 could be a potential target for developing new diabetes treatments.
- Differences Between Cell Types: The study also highlighted the differences in how various pancreatic cell types respond to stress. For instance, α-cells and ductal cells exhibited different patterns of gene expression under stress compared to β-cells. Understanding these differences is crucial for developing targeted therapies that address the specific needs of each cell type.
Implications for Diabetes Treatment:
The findings from this study provide a detailed map of how pancreatic cells react to stress, offering insights that could lead to new therapeutic strategies. By targeting specific stress pathways, it may be possible to protect β-cells from damage and improve the overall health of pancreatic islets in diabetic patients.
About the Researchers:
This research was conducted by a team of scientists including Marlie Maestas, Dr. Jeffrey Millman, Dr. Fumihiko Urano, and their colleagues at Washington University School of Medicine. Their work continues to advance our understanding of diabetes and pave the way for innovative treatments.
For more detailed information, you can access the full study here.
References: Maestas, M. M., Ishahak, M., Augsornworawat, P., Veronese-Paniagua, D. A., Maxwell, K. G., Velazco-Cruz, L., Marquez, E., Sun, J., Shunkarova, M., Gale, S. E., Urano, F., & Millman, J. R. (2024). Identification of unique cell type responses in pancreatic islets to stress. Nature Communications, 15:5567. https://doi.org/10.1038/s41467-024-49724-w.
Dr. Millman Promoted
By Erika Brown – July 1, 2024

Dr. Millman received a promotion to full Professor today. Congratulations, Dr. Millman!
Dr. Millman Presents at ADA
By Erika Brown – July 1, 2024

Jeffrey Millman, PhD, presented at the American Diabetes Association’s (ADA) 84th Scientific Sessions this June in Orlando, FL. This prestigious meeting convenes experts in diabetes research, treatment, and care to share the latest findings and discuss advancements in the field. It serves as a crucial platform for exchanging new insights, fostering collaborations, and shaping the future of diabetes management worldwide.
In his presentation, Dr. Millman provided an update on the progress in creating and understanding stem cell-derived islets. He highlighted his lab’s latest work, which includes sophisticated bioinformatic modeling of the entire differentiation process at a single-cell level, both transcriptionally and epigenetically.
On Thursday, June 20, Dr. Millman participated in an expert panel discussing the heterogeneity of type 1 diabetes (T1D). The panel covered topics such as beta cell pathology, T1D prevention, and insulin replacement. Dr. Millman was also one of the experts selected to present a summary of the panel discussion to the meeting attendees on Monday, June 24. Additionally, he joined a public panel to delve deeper into the heterogeneity of T1D. Following these discussions, Dr. Millman will be co-authoring a review article with the other panel participants to further disseminate their findings to the broader scientific community.
Tai Le Joins the Lab
By Erika Brown – June 18, 2024

Tai Le joined the lab as a Research Technician this week. He recently obtained his B.S. in Cell and Molecular Biology from Missouri State University, and plans on pursuing an M.D./Ph.D. in the future. Outside of lab, Tai enjoys cooking, writing, and reading anything relating to literature and philosophy. Welcome, Tai!
Dr. Millman Featured in DOM News
May 22, 2024

Dr. Millman was the subject of an article written recently for the Department of Medicine. The article spotlighted the research at the Millman Lab and some of Dr. Millman’s background in diabetes research. Read the article here.
Dr. Ishahak at Fellows’ Research Day
May 2, 2024

Dr. Matthew Ishahak presented at the division Fellows’ Research Day. His talk was titled “Single-Cell Multiomic Atlas Elucidates Cell Fate Specification in Stem Cell-derived Islets.” Fellows’ Research Day is a twice-per-year opportunity for fellows in the department to showcase their research.
Millman Lab at the Dream Gala St. Louis
April 18, 2024
Members of the Millman Lab and their families attended the 2024 JDRF Dream Gala St. Louis on Saturday. The gala is a fundraising event for community and corporate leaders in the T1D community benefiting the JDRF Kansas & Missouri chapter.
Clinical Trial Featuring Millman Lab Research Gets Update
April 15, 2024
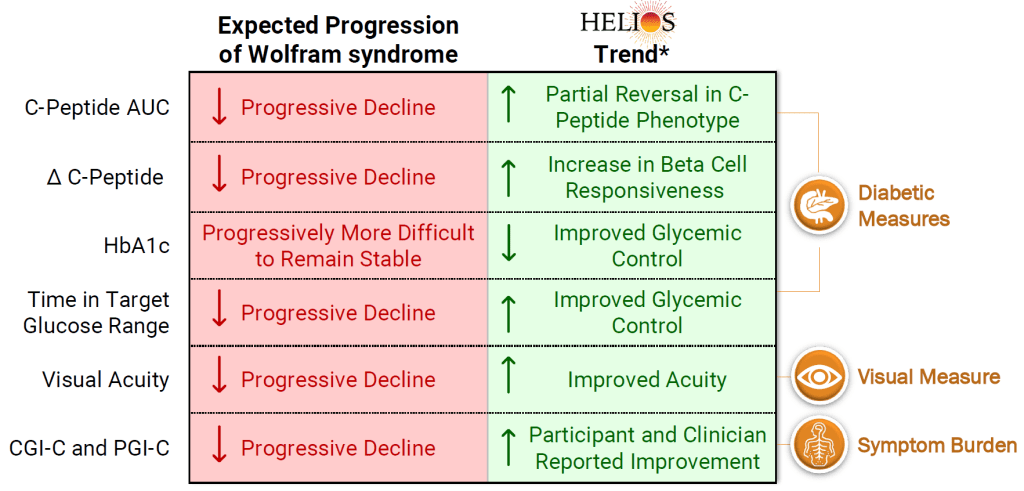
Last week, Amylyx Pharmaceuticals released interim data from the ongoing HELIOS clinical trial that features Millman Lab research. The trial is using AMX0035 to improve pancreatic function and glycemic control in Wolfram Syndrome patients. The Millman Lab previously used SC-islets that were made from patients with Wolfram Syndrome to do a preclinical evaluation of this compound. The interim data showed that patients had an increase in C-peptide and beta cells had more rapid responses to glucose challenge, which was unexpected due to disease progression. Additionally, most patients had a decrease in A1c and an increase in time in range. There were other benefits as well reflected in the summary graphic above. Read more about the preclincal study here and the update here.
Dr. Millman Featured On The Sugar Science Podcast
March 5, 2024
Dr. Millman was featured in the “Ask the Expert” segment of The Sugar Science podcast. He discussed using single-cell sequencing to understand the cellular complexity of SC-Islet identity.
Ella and Liam Join the Lab
February 19, 2024
Ella Hanson and Liam Oiknine joined the lab as trainees this month. Ella is in the B.S./M.S. Biomedical Engineering program at WashU and Liam is a Freshman at WashU, studying Computer Science and Biology. Welcome, Ella and Liam!
New Paper in BMC Genomics
January 24, 2024
Mason Schmidt (former postbacc student), Matt Ishahak, Punn Augsornworawat (former Ph.D. student) and Dr. Millman published a paper in BMC Genomics. The publication reveals comparisons of SC-islets, fetal islets, and adult islets using single-cell sequencing datasets to characterize the transcriptional identity of each. The team’s analysis revealed that SC-β cells share a core β-cell transcriptional identity with human adult and fetal β-cells, however SC-β cells possess a unique transcriptional profile. Read the open-access article here.
2023 Holiday Party
December 12, 2023
The Millman Lab celebrated the holidays and the end of the year with an afternoon at Topgolf in St. Louis. Trainees and staff played a few games and white elephant gifts were exchanged.
Happy holidays from the Millman Lab!
Millman Lab Recognized at the COVID-19 Awards
November 28, 2023

Drs. Millman and Hogrebe were recognized at the WashU COVID-19 Awards. The COVID-19 Awards recognize outstanding individuals for their work and contributions in the Department of Medicine during the pandemic.
Dr. Millman was awarded the COVID Star Award and Dr. Hogrebe was awarded the COVID Rising Star Award.
Congratulations, Drs. Millman and Hogrebe!
Millman Lab Receives Green Lab Certification at Green Carpet Awards
 November 21, 2023
November 21, 2023
The Green Carpet Awards are an annual award ceremony honoring WashU labs and offices that certified as Sustainability Champions through the WashU Sustainability office. The Millman Lab was certified at the Gold level for its efforts to conduct research in more sustainable ways. Erika Brown (back right) attended the awards as the lab’s Green Lab representative. Read more about the awards here and the Green Labs program here.
Millman Lab Participates in 2023 Diabetes Day Symposium
November 9, 2023
The WashU Diabetes Research Center hosts an annual diabetes symposium in honor of World Diabetes Day, which falls on November 14. The event brings together DRC scientists and their trainees to showcase the latest developments in diabetes research. During the symposium, Dr. Lori Sussel, Research Director of the Barbara Davis Center for Diabetes at the University of Colorado, delivered a lecture. Danny Veronese Paniagua, Ed Castro-Sanchez, Kameron Bradley, Marlie Maestas, and Matt Ishahak presented posters throughout the day-long event.
Dr. Millman Speaks at 50th Kilo Diabetes Symposium

Dr. Millman spoke at the 50th annual Kilo Diabetes Symposium on Saturday. He gave a talk about Cell Therapy for T1D and participated in a panel discussion on T1D Prevention and Reversal.
The Kilo Diabetes Symposium is a national forum for leaders in the field to educate the medical community about research findings and good clinical practice in diabetes, endocrinology, and cardiovascular diseases.
Millman Lab at 2023 JDRF One Walk
Several members of the Millman Lab and their families came out to the 2023 JDRF One Walk. The one-mile walk is a fundraiser for diabetes research and a way for those living with T1D and their friends and families to come together and support the fight for a cure. It was a beautiful day to spend together and support our friends at JDRF!
Matt and Kameron Present at BMES 2023
Dr. Matt Ishahak and Kameron Bradley spent last week at the 2023 Biomedical Engineering Society (BMES) Annual Meeting in Seattle, WA. Kameron presented a poster on his hypoxia work and Matt gave a talk on the lab’s HIRN catalyst project. Matt also received an award from the Engineering Endocrine Tissue Special Session.
Millman Lab at Environmental Justice Day of Action

Dr. Millman, Charlotte Millman, Alexa Wagner, Matt Ishahak, and Erika Brown participated in a neighborhood cleanup in O’Fallon Park in North City St. Louis on Saturday. The cleanup was part of St. Louis Environmental Justice Days of Action, an initiative to address environmental justice issues such as illegal dumping and food apartheid, which is led by earthday365 and North Newstead Association. It was a great day to help out the local community!
Alexa Joins the Lab

Alexa Wagner joins the lab as a Research Assistant. Welcome, Alexa!
Dr. Ishahak Wins Award

Dr. Matt Ishahak received the Distinguished Postdoctoral Scholars award from the Center of Regenerative Medicine. This program is designed to develop highly trained individuals who can produce impactful research in regenerative medicine through an interdisciplinary training plan. Congratulations, Matt!
Drs. Millman and Ishahak Attend HIRN Annual Meeting
Dr. Millman and Dr. Matt Ishahak attended the Human Islet Research Network (HIRN) annual meeting last week. They both presented at the meeting and Dr. Millman participated in the hypoimmune islet panel.
Millman Lab at Environmental Justice Day of Action

Dr. Millman, Charlotte Millman, Ed Castro-Sanchez, Marlie Maestas, and Erika Brown participated in a neighborhood cleanup in Jennings, MO on Saturday. The cleanup was part of St. Louis Environmental Justice Days of Action, an initiative to address environmental justice issues such as illegal dumping and food apartheid, which is led by earthday365. It was a great day to spend some time together and do some good for the community!
Cam’s Last Day

The Millman Lab said goodbye to Cameorn Banks on Friday with lunch from Lona’s Lil Cafe. Cam is heading to a Ph.D. program at the University of Minnesota in the fall where he will study within the Microbiology, Immunology, and Cancer Biology (MICaB) program. Good luck, Cam; we will miss you!
Veronica’s Last Day

Veronica Lee had her last day of her summer research period in the lab on Wednesday. She will continue her undergraduate study at WashU. Good luck, Veronica!
Diana Promoted

Diana Hernandez has been promoted to Senior Research Technician. Congratulations, Diana!
Camryn Gives Talk at uSTAR Closing Symposium

Camryn Moore spoke on her research in the Millman Lab at the uSTAR Summer Scholars Program Closing Symposium. The talk was entitled “CRISPR Validation and Functional Analysis of Hypoxic and Normoxic Stem Cell-Derived Islets.” Great job, Camryn!
JDRF Kids Visit Millman Lab
The Millman Lab had a recent visit from the local chapter of JDRF, where we had the pleasure of hosting several families with children affected by T1D. Guests were given a tour of the lab with talks by Millman Lab members. A reception followed with pipette racing, a sink or float experiment, and a photo booth. Thank you to Millman Lab volunteers, JDRF, and the families!
Lab Certifies Gold With WashU Green Labs

The Millman Lab has been certified gold with WashU’s Green Labs Program. Read More about some of our sustainability efforts here!
Mason Farewell Lunch

The Millman Lab said goodbye to Mason Schmidt on Monday with lunch from Gioia’s Deli. Mason is heading to a Ph.D. program at Duke in the fall where he will study within the Developmental and Stem Cell Biology program. Good luck, Mason; we will miss you!
Dr. Millman Speaks at 2023 Endocrine Society
Dr. Millman spoke at ENDO 2023 in Chicago over the weekend. His talk was featured at the plenary session on “The Future of Endocrine Therapies: Personalized Organoids And Gene Editing – Is This The Real-Life or Just Fantasy?”.
Millman Lab Presents at ISSCR

Kameron Bradley, Danny Veronese Paniagua, Matt Ishahak, Diana Hernandez, and Marlie Maestas attended the 2023 International Society for Stem Cell Research (ISSCR) meeting in Boston, MA. They presented posters on the latest research from the lab.
Millman Lab Featured in WashU News

The Millman Lab’s latest publication in Nature Cell Biology was featured in a press release by WUSM News. The article explains the study, which was on creating improved stem cell-derived beta cells through machine learning and computational methods. Read the article here.
Camryn and Veronica Join the Lab
Camryn Moore and Veronica Lee both join the lab as undergraduate trainees. Camryn is with the WashU U-Star Summer Scholar Program and Veronica is with the WashU Amgen Scholars Program. They will be mentored by Kameron Bradley and Marlie Maestas respectively. Welcome, Camryn and Veronica!
Allison Joins the Lab

Allison Kelley joins the lab as a Research Technician. Welcome, Allison!
New Paper in Nature Cell Biology

Link to the open access article here.
Diana Graduates
Ed Joins the Lab

New Paper in Nature Biotechnology

Link to the open access article here.
Lab Participates in Park Cleanup

Members of the Millman Lab and their families participated in a cleanup of O’Fallon Park in St. Louis. The cleanup was part of St. Louis Environmental Justice Days of Action, an initiative to address environmental justice issues such as illegal dumping and food apartheid. It was a great day to spend some time together and do some good for the community.
New Paper in Cell Stem Cell

Link to the open access article here.
Millman Lab Lunch

Dr. Millman Study Featured on JDRF News

Aining Passes Masters Thesis Defense

Chandler Accepts Position at Bayer

Mira Joins the Lab

Mason Awarded NSF Fellowship

Punn Defends Thesis

Matt Accepts Position On WashU Council

Danny Receives Award

Cameron Accepted Into Graduate School

JDRF Visit
Nathaniel Hogrebe Promoted

Lab Holiday Party

Nathaniel Hogrebe Presented at GESC Symposium

Erika joins the Millman lab

Punn Augsornworawat receives award on Diabetes day

Daniel Veronese-Paniagua at SACNAS NDiSTEM

Punn Augsornworawat, Matthew Ishahak, and Aining Fan at BMES 2022


Punn Augsornworawat at TERMIS-AP 2022 South Korea

New paper on Advanced Materials Technologies

New paper on JCI Insight

Millmab lab at DBBS Retreat



Matthew Ishahak presented at UNITE

Danny is part of E4B

Jeffrey Millman receives HIRN grant

Punn Augsornworawat featured on The Scientist


Jeffrey Millman named Mallinckrodt Scholar

Dr. Millman on AP news
July 19, 2022
![]()
Jeffrey Millman, PhD. was recently interviewed by the Associated Press on growing stem cells in space. This topic covers interesting areas on whether or not stem cells can grow in the zero gravity condition. Check out the this link here.
Matthew awarded by NIH
 Matthew Ishahak was selected for the NIH National Research Service Award (NRSA) postdoctoral fellowship on the Diabetes and Related Metabolic Diseases Training grant in the Division of Endocrinology at Washington University School of Medicine (5 T32 DK007120)
Matthew Ishahak was selected for the NIH National Research Service Award (NRSA) postdoctoral fellowship on the Diabetes and Related Metabolic Diseases Training grant in the Division of Endocrinology at Washington University School of Medicine (5 T32 DK007120)Millman lab team presents at ISSCR 2022
June 18, 2022

Mason joins dkNET Summer of Data Student Program
June 2, 2022

Mason Schmidt, our lab’s postgraduate researcher, joins the 2022 dkNET Summer of Data Student Program and is awarded a scholarship for the program. Congratulations!
Julia Miller graduates from Washu!
June 1, 2022

Julia Miller graduated with a Bachelor of science in biomedical engineering this summer. She has been actively working with the Millman Lab on engineering Stem cells to produce pancreatic islets. Congratulations!
Augsornworawat wins BME research award
May 11, 2022

Punn Augsornworawat received the Biomedical Engineering (BME) Outstanding Research Award on May 2, 2022. His works are focused on developing methodologies to generate pancreatic islets from stem cells and utilizing single-cell technologies to study islet development and β-cell maturation.
Maggie joins the Lab!
April 4, 2022

We are proud to announce that Maggie Bui, MSTP student, will be joining our lab to work on her PhD in islet development and biology.
New publication on Stem Cells Translational Medicine
March 21, 2022
We are proud to share our new publication “Differential Function and Maturation of Human Stem Cell-Derived Islets After Transplantation”. This study explores different transplantation strategies for SC-islets in diabetic mice. Link

“A Possible Cure for Diabetes”
February 1, 2022
Dr. Millman is featured in Eureka’s Podcast “A Possible Cure for Diabetes”. Check out on our research highlights and current progress in Diabetes cure. Link 
Millman lab welcomes new members!
Janurary 20, 2022
We are excited to welcome 2 new members to the Millman Lab. Kameron joined us as a doctoral student from the department of biomedical engineering. Aining, from the department of biomedical engineering, joined us a Masters student. Welcome!


Dr. Millman was interviewed for an article in IEEE Pulse
November 9, 2021
Dr. Millman was interviewed for an article in IEEE Pulse about the promises and challenges of stem cells for diabetes cell replacement therapy. Check out the article here!
Julia Receives Future Leaders in Chemical Engineering Award
November 8, 2021
We are proud to see Julia Miller, an Undergraduate student in Biomedical Engineering who has been working with us, receiving a Future Leaders in Chemical Engineering award! Julia’s work is on genetic engineering of Stem cell derived Islets. Congratulations Julia!

Dr. Est joins Millman lab!
October 18, 2021
Millman lab welcomes new Post-doctoral fellow Dr. Chandler Est! Chandler will be studying Islet differentiations using genetic engineering tools and developing new approaches to investigate diabetes.

Millman lab presents at BMES 2021 in Orlando
October 15, 2021
Last week, Punn Augsornworawat, Matthew Ishahak, and Julia Miller gave oral presentations at the 2021 BMES conference in Orlando, Florida. Each presented their Stem cell and Diabetes research work.

Dr. Millman is featured on an interview with Nature
September 29, 2021
Nature Publishing Group interviewed Dr. Millman on the feasibility and current efforts in scaling and manufacturing cells for cell replacement therapies. The interview is featured in “Stem-cell start-ups seek to crack the mass-production problem”
Matthew receives NIH LRP award
August 6, 2021
Big congratulations to Dr. Ishahak for receiving the NIH LRP award from the National institute of Diabetes and Digestive and kidney diseases (NIDDK).

Published in Nature Protocols!
August 3, 2021
Our strategy for making islets from ES/iPS cells is now out in Nature Protocols. In this article, Co-first authors Hogrebe, Maxwell, and Augsornworawat illustrate making islets from patient iPSCs (T1D, T2D, MODY, Wolfram Syndrome). We hope that this is a useful resource for other labs.

Congratulations Dr. Leo!
July 27, 2021
Leonardo defended his PhD Thesis on the functional development and maturation of SC-Beta cells. Huge congrats to Dr. Velazco-Cruz!

New Paper on Oxygen Effect on Stem Cell Differentitations
July 12, 2021
Dr. Millman publishes new research article on the effects of physiological oxygen on teratoma formation in Stem cell differentiations
Free access to the article can be found found here: Link
Review Paper on Islet Encapsulation Published
June 22, 2021
Dr. Millman publishes a review article on designing encapsulation devices using SC-Islets for T1D treatment.
New Publication in Science Translational Medicine
June 2, 2021
A team of researchers led by diabetes specialists and biomedical engineers at Washington University School of Medicine in St. Louis and Cornell University has demonstrated that, using a miniscule device, they can implant insulin-secreting cells into diabetic mice. Once implanted, the cells secrete insulin in response to blood sugar, reversing diabetes without requiring drugs to suppress the immune system.

The findings are published June 2 in the journal Science Translational Medicine.
*Portions of this originally published by Washington University School of Medicine
Dr. Millman receives tenure!
May 14, 2021
Dr. Jeffrey Millman has been promoted to Associated Professor and granted tenure effective June 1!

Michelle joins Duke University for the MD Program
May 7, 2021
Michelle joined the Millman lab in 2017 and made huge contributions to our work in Diabetes Research. She will now be joining the MD program at Duke University. We bid her farewell and wish her great successes in her future endeavors!
Millman lab members win company pitch competition
April 27, 2021
Millmab Lab members, Leo, Punn, Kristina and Dr. Millman, led Salentra Biosciences is awarded $7500 in funding from the Skandalaris Venture competition.

New review on IPS derived Beta cell applications
April 20, 2021
Dr. Maxwell and Dr. Millman publishes a new review article on the applications of IPS derived Beta cells!

Marlie joins the Millman Lab!
January 29, 2021
Marlie Maestas joins the Millman Lab as a doctoral student from the DBBS program. Welcome Marlie!

Millman Lab Welcomes new members!
February 14, 2021
The Millman Lab is proud to have additional members join our team. Sarah Gale joins us as our senior lab manager. Diana and Cameron are here to join us as Lab technicians. The two will be conducting research in optimizing and expanding in vitro Islet generation for diabetes cell replacement therapy.



Dr. Millman Received CMBE Award
January 2, 2021
Dr. Jeffrey Millman was named and awarded the 2021 Cellular and Molecular Bioengineering (CMBE) Rising Star by BMES. Congratulations!

Congratulations Dr. Maxwell
December 7, 2020
Big congrats to Millman lab Doctoral student, Kristina Maxwell for successfully defending her thesis entitled “Autologous Stem Cell-Derived β Cells for Diabetes Cell Replacement Therapy”. Her work has provided significant contributions to the diabetes cell replacement therapy field. Congratulations Dr. Maxwell!

Millman Lab participates JDRF One Walk
October 31, 2020
Millman Lab team participates this year’s JDRF One Walk fundraising event. The “Beta Squad” team raised funds and enjoyed the St. Louis’s forest park stroll to support research in curing Type I Diabetes.

Millman Lab at BMES 2020
October 14, 2020
Millman Lab team, Punn Augsornworawat, Kristina Maxwell and Dr. Jeffrey Millman himself will be giving talks at the 2020 BMES Annual meeting. Please check out their talks and feel free to drop by for the live Q&A sessions.
Kristina Maxwell – Stem Cell Engineering I October 15 3PM-4PM
Jeffrey Millman – Stem Cell Engineering II October 15 9AM-10AM
Punn Augsornworawat – Stem Cell Engineering II October 15 9AM-10AM



Dr. Millman featured on Podcast
September 19, 2020
Dr. Jeffrey Millman spoke at a podcast on the development and advancements of Stem cells for treating type I diabetes.

Check it out from these links:
Apple Podcasts bit.ly/JBPAPod
Spotify bit.ly/JBPspot
Amazon Music bit.ly/JBPAmazonMusic
Online bit.ly/JuiceboxPod
Leo is awarded NIH F31
August 26, 2020
 Congratulations to Millman Lab Doctoral student Leonardo Velazco-Cruz for getting the prestigious NIH F31 Fellowship! His excellent achievements have proven him worthy of this award. The team is proud of his accomplishments.
Congratulations to Millman Lab Doctoral student Leonardo Velazco-Cruz for getting the prestigious NIH F31 Fellowship! His excellent achievements have proven him worthy of this award. The team is proud of his accomplishments.
August 25, 2020
 Millman lab’s recent paper, Single-Cell Transcriptome Profiling Reveals β Cell Maturation in Stem Cell-Derived Islets after Transplantation, is featured on the Cell Reports Journal cover! Check out the released journal issue on the link below.
Millman lab’s recent paper, Single-Cell Transcriptome Profiling Reveals β Cell Maturation in Stem Cell-Derived Islets after Transplantation, is featured on the Cell Reports Journal cover! Check out the released journal issue on the link below.
August 25, 2020

Stem Cell-Derived Islets Now Resemble Human Islets
Researchers at Washington University have been generating insulin-producing islets in the laboratory from human stem cells. This approach promises a virtually unlimited supply of replacement insulin-producing tissues to functionally cure patients with diabetes. However, cell-derived islets are immature compared to islets from the body, lacking the expression of genes associated with maturation.
Here, a team of researchers from the laboratory of Dr. Jeffrey Millman at Washington University in St. Louis have discovered that these stem cell-derived islets are capable of maturing after transplantation to resemble real islets more closely from humans. The team, with co-first authors Punn Augsornworawat and Kristina Maxwell, accomplished this feat by developing a new methodology to isolate transplanted cells and performed single-cell RNA sequencing to catalogue these changes in individual cells.
The mice used in the experiments had severe diabetes. After several months, these mice were completely cured of diabetes, with the transplanted stem cell derived islets providing robust insulin release and blood glucose regulation, equivalent to healthy mice.
These long-term improvements in function are associated with increased expression of mature genes, such as MAFA and G6PC2. In addition, the team also identified other mature markers including FAM159B, and MT1X, to robustly confirm maturation and found that secretion of the peptide IAPP to be a detectable biomarker for maturation more robustly.
These findings highlight the similarities between lab-grown islets after transplantation and native islets from the body and provides a new technological pipeline allowing for more rigorous study of cellular maturation of differentiated cells from stem cells. The research article was published on August 25, 2020 in the journal of Cell Reports.
This study was supported by the National Institutes of Health (5R01DK114233, T32DK108742, P30CA91842, UL1TR000448), a JDRF Career Development Award (5-CDA-2017-391-A-N), Washington University-Centene Personalized Medicine Initiative, David and Deborah Winston Fellowship in Diabetes Research, and joint funds from Washington University (Departments of Surgery, Medicine, and Pediatrics), Mid-America Transplant Services, and The Foundation for Barnes-Jewish Hospital.
New review article on single-cell technologies
August 4, 2020

Punn Augsornworawat and Dr. Millman published a review article on Single-cell RNA sequencing to investigate pancreas development and diabetes.
Leo and Maddy published new article
July 9, 2020


Leonardo Velazco-Cruz and Madeleine Goedegebuure published a review article on β cell development and functional maturation. Congratulations!
Millman lab PhD students win travel awards
Matt receives Rita Levi-Montalcini Postdoctoral Fellowship
July 1, 2020
Congratulations to our Postdoctoral Fellow, Dr. Matthew Ishahak, for receiving the first Rita Levi-Montalcini Postdoctoral Fellowship in Regenerative Medicine!

Millman lab welcomes new members!
June 15, 2020
We are pleased to welcome Erica Marquez, and the Rita Levi-Montalcini postdoctoral fellow Dr. Matthew Ishahak to join our team. Dr. Ishahak will be using his microfluidic expertise to design devices to model and study the behaviors of stem cell-derived islets. Ms. Marquez will explore strategies to genetically engineer stem cell-derived islets for studying diabetes. We look forward to their future contributions to the diabetes therapy field.
New publication in Cell Reports
May 26, 2020

Congratulations to Leo, Maddy, and the rest of the team for their new publication in Cell Reports! In it, they discovered that the transcription factor SIX2 is essential for the functional maturation of stem cell-derived beta cells. This is important as our team continues to engineer these cells for diabetes cell replacement therapy.
Generation of insulin-secreting β cells in vitro is a promising approach for diabetes cell therapy. Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) are differentiated to β cells (SC-β cells) and mature to undergo glucose-stimulated insulin secretion, but molecular regulation of this defining β cell phenotype is unknown. Here, we show that maturation of SC-β cells is regulated by the transcription factor SIX2. Knockdown (KD) or knockout (KO) of SIX2 in SC-β cells drastically limits glucose-stimulated insulin secretion in both static and dynamic assays, along with the upstream processes of cytoplasmic calcium flux and mitochondrial respiration. Furthermore, SIX2 regulates the expression of genes associated with these key β cell processes, and its expression is restricted to endocrine cells. Our results demonstrate that expression of SIX2 influences the generation of human SC-β cells in vitro.
Congratulations to Maddy for graduating!
May 22, 2020
 Maddy will be moving on to do a PhD at Northwestern. We will miss you!
Maddy will be moving on to do a PhD at Northwestern. We will miss you!
Diabetes reversed in mice with genetically edited stem cells derived from patients – new publication in Science Translational Medicine
April 22, 2020
Using induced pluripotent stem cells produced from the skin of a patient with a rare, genetic form of insulin-dependent diabetes called Wolfram syndrome, researchers transformed the human stem cells into insulin-producing cells and used the gene-editing tool CRISPR-Cas9 to correct a genetic defect that had caused the syndrome. They then implanted the cells into lab mice and cured the unrelenting diabetes in those mice.
The findings, from researchers at Washington University School of Medicine in St. Louis, suggest the CRISPR-Cas9 technique may hold promise as a treatment for diabetes, particularly the forms caused by a single gene mutation, and it also may be useful one day in some patients with the more common forms of diabetes, such as type 1 and type 2.
The study is published online April 22 in the journal Science Translational Medicine.
Patients with Wolfram syndrome develop diabetes during childhood or adolescence and quickly require insulin-replacement therapy, requiring insulin injections multiple times each day. Most go on to develop problems with vision and balance, as well as other issues, and in many patients, the syndrome contributes to an early death.
“This is the first time CRISPR has been used to fix a patient’s diabetes-causing genetic defect and successfully reverse diabetes,” said co-senior investigator Jeffrey R. Millman, PhD, an assistant professor of medicine and of biomedical engineering at Washington University. “For this study, we used cells from a patient with Wolfram syndrome because, conceptually, we knew it would be easier to correct a defect caused by a single gene. But we see this as a stepping stone toward applying gene therapy to a broader population of patients with diabetes.”
Wolfram syndrome is caused by mutations to a single gene, providing the researchers an opportunity to determine whether combining stem cell technology with CRISPR to correct the genetic error also might correct the diabetes caused by the mutation.
A few years ago, Millman and his colleagues discovered how to convert human stem cells into pancreatic beta cells. When such cells encounter blood sugar, they secrete insulin. Recently, those same researchers developed a new technique to more efficiently convert human stem cells into beta cells that are considerably better at controlling blood sugar.
In this study, they took the additional steps of deriving these cells from patients and using the CRISPR-Cas9 gene-editing tool on those cells to correct a mutation to the gene that causes Wolfram syndrome (WFS1). Then, the researchers compared the gene-edited cells to insulin-secreting beta cells from the same batch of stem cells that had not undergone editing with CRISPR.
In the test tube and in mice with a severe form of diabetes, the newly grown beta cells that were edited with CRISPR more efficiently secreted insulin in response to glucose. Diabetes disappeared quickly in mice with the CRISPR-edited cells implanted beneath the skin, and the animals’ blood sugar levels remained in normal range for the entire six months they were monitored. Animals receiving unedited beta cells remained diabetic. Their newly implanted beta cells could produce insulin, just not enough to reverse their diabetes.
“We basically were able to use these cells to cure the problem, making normal beta cells by correcting this mutation,” said co-senior investigator Fumihiko Urano, MD, PhD, the Samuel E. Schechter Professor of Medicine and a professor of pathology and immunology. “It’s a proof of concept demonstrating that correcting gene defects that cause or contribute to diabetes — in this case, in the Wolfram syndrome gene — we can make beta cells that more effectively control blood sugar. It’s also possible that by correcting the genetic defects in these cells, we may correct other problems Wolfram syndrome patients experience, such as visual impairment and neurodegeneration.”
In the future, using CRISPR to correct certain mutations in beta cells may help patients whose diabetes is the result of multiple genetic and environmental factors, such as type 1, caused by an autoimmune process that destroys beta cells, and type 2, which is closely linked to obesity and a systemic process called insulin resistance.
“We’re excited about the fact that we were able to combine these two technologies — growing beta cells from induced pluripotent stem cells and using CRISPR to correct genetic defects,” Millman said. “In fact, we found that corrected beta cells were indistinguishable from beta cells made from the stem cells of healthy people without diabetes.”
Moving forward, the process of making beta cells from stem cells should get easier, the researchers said. For example, the scientists have developed less intrusive methods, making induced pluripotent stem cells from blood — and they are working on developing stem cells from urine samples.
“In the future,” Urano said, “we may be able to take a few milliliters of urine from a patient, make stem cells that we then can grow into beta cells, correct mutations in those cells with CRISPR, transplant them back into the patient, and cure their diabetes in our clinic. Genetic testing in patients with diabetes will guide us to identify genes that should be corrected, which will lead to a personalized regenerative gene therapy.”
[portions of this was taken from the WashU press release by Jim Dryden]
New patent issued
April 17, 2020
The team just had a new patent issued for our invention of using 3D printing to make a transplantable scaffold for stem cell-derived islets.
Jeffrey R. Millman and Jiwon Song. 3D-printed scaffold device for cell transplantation. 10,597,639. Issued March 24, 2020
Danny awarded fellowship from the NSF

March 29, 2020
Danny has received a NSF Graduate Research Fellowship Program (GRFP) fellowship. This award “recognizes and supports outstanding graduate students in NSF-supported science, technology, engineering, and mathematics disciplines who are pursuing research-based Master’s and doctoral degrees at accredited United States institutions”, according the NSF website.
Danny joins the lab!

March 23, 2020
We are excited to have Danny join the lab as a PhD student in the DBBS program. He will be studying the molecular mechanisms that control cellular fate and health.
New recent fellowships!


February 26, 2020
Congratulations to Dr. Nathaniel Hogrebe and Punn Augsornworawat for receiving the JDRF Advanced Postdoctoral Fellowship and David and Deborah Winston Fellowship in Diabetes Research Award, respectively!
Diabetes in mice cured rapidly using human stem cell strategy

February 24, 2020
Researchers in the Millman lab have converted human stem cells into insulin-producing cells and demonstrated in mice infused with such cells that blood sugar levels can be controlled and diabetes functionally cured for nine months.
The findings, from researchers at Washington University School of Medicine in St. Louis, are published online Feb. 24 in the journal Nature Biotechnology.
“These mice had very severe diabetes with blood sugar readings of more than 500 milligrams per deciliter of blood — levels that could be fatal for a person — and when we gave the mice the insulin-secreting cells, within two weeks their blood glucose levels had returned to normal and stayed that way for many months,” said principal investigator Jeffrey R. Millman, PhD, an assistant professor of medicine and of biomedical engineering at Washington University.
Several years ago, the same researchers discovered how to convert human stem cells into pancreatic beta cells that make insulin. When such cells encounter blood sugar, they secrete insulin. Still, previous work has had its limitations and had not effectively controlled diabetes in mice.
Now, the researchers have shown a new technique they developed can more efficiently convert human stem cells into insulin-producing cells that more effectively control blood sugar.
“A common problem when you’re trying to transform a human stem cell into an insulin-producing beta cell — or a neuron or a heart cell —is that you also produce other cells that you don’t want,” Millman said. “In the case of beta cells, we might get other types of pancreas cells or liver cells.”
Off-target pancreas and liver cells don’t hurt anything when implanted into a mouse, but they don’t fight diabetes either.
“The more off-target cells you get, the less therapeutically relevant cells you have,” he said. “You need about a billion beta cells to cure a person of diabetes. But if a quarter of the cells you make are actually liver cells or other pancreas cells, instead of needing a billion cells, you’ll need 1.25 billion cells. It makes curing the disease 25% more difficult.”
Using the new technique, Millman’s team found far fewer off-target cells were produced while the beta cells that were made had improved function. The technique targets the cells’ internal scaffolding, called the cytoskeleton. The cytoskeleton is what gives a cell its shape and allows the cell to interact with its surrounding environment, converting physical cues into biochemical signals.
“It’s a completely different approach, fundamentally different in the way we go about it,” he said. “Previously, we would identify various proteins and factors and sprinkle them on the cells to see what would happen. As we have better understood the signals, we’ve been able to make that process less random.”
Understanding that process has allowed Millman’s team to produce more beta cells. Importantly, the new technique works efficiently across stem cells from multiple different sources, greatly expanding the ability of this technique in the study of disease.
“We were able to make more beta cells, and those cells functioned better in the mice, some of which remained cured for more than a year,” Millman said. “
He explained that there still is much to do before this strategy can be used to treat people with diabetes. They will need to test the cells over longer periods of time in larger animal models and work to automate the process to have any hope of producing beta cells that can help the millions of people who currently require insulin injections to control their diabetes. But the research is continuing.
Portions of this is from the WashU press release linked below:
Celebration dinner!

February 4, 2020
The lab celebrated a couple of paper acceptances, a new fellowship, and several positive reviews on new grants and paper. Great way to start the year off!
Welcome Julia!

January 17, 2020
We welcome Julia into the lab as an undergraduate researcher. She is working with Punn on studying islet heterogeneity.
Welcome Shreya!

January 13, 2020
We welcome Shreya into the lab as an undergraduate researcher. She is working with Kristina on in vitro models of type 2 diabetes.
Congratulations to Michelle for graduating this December

January 8, 2020
Congratulations to Michelle for graduating with her B.S. in Biochemistry from WashU this December!
Dr. Millman awarded a Distinguished Young Alumni Award

January 7, 2020
Dr. Millman was honored with a Distinguished Young Alumni Award from the Chemical and Biomolecular Engineering (CBE) Department at North Carolina State University, the highest award that CBE can bestow on alumni. This honor recognizes the achievements and contributions of alumni to their profession and serves as an inspiration for current and future students.
Holiday lab lunch!

December 13, 2019
Many reason for us to celebrate this year. New papers, grants, fellowships, award, and graduation! Such a wonderful team!
Labsgiving!

November 25, 2019
The lab celebrated “Labsgiving” this week. Many things to be grateful for!
Madeleine Goedegeburre named a Future Leader in Chemical Engineering

October 23, 2019
Maddy was chosen as a Future Leader in Chemical Engineering from North Carolina State University based on her research in the lab engineering stem cells for the study and treatment of diabetes. Congratulations!
Millman lab presents at this the annual BMES meeting

October 17, 2019
Punn and Maddy presented posters on their work studying differentiation fate and developing screening approaches for diabetes. Also encountered Millman lab alumna Jiwon!
New platform for the assembly of islet organoids from stem cells

August 22, 2019
Co-first authors Punn Augsornworawat and Leonardo Velazco-Cruz published an article entitled “A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells” in Acta Biomaterialia. We also congratulate Punn on his first first-author publication! We hope this platform will enable fabrication of advanced regenerative medicine products for diabetes therapy.
Millman lab presents at Oxford and EASD!


August 5, 2019
Jeff and Leo presented at the EASD-Hagedorn Oxford Workshop on the latest protocol for generating functional beta cells for therapy.
Maddy received an Amgen Foundation Scholarship

July 17, 2019
Maddy received an Amgen Scholarship to continue performing research in the Millman lab with stem cells and genetic engineering – congratulations!
Millman lab presents at the annual ISSCR meeting!

June 26, 2019
Kristina presented at the International Society for Stem Cell Research on her work with differentiated patient-derived stem cells
Arvind Srivatsava defends his M.S. thesis

May 8, 2019
Congratulations to Arvind Srivatsava for defending his Biomedical Engineering Master’s Thesis on the microfluidic assessment of stem cell-derived beta cells!
Many Congratulations!
 May 7, 2019
May 7, 2019
Kristina Maxwell received the Greg Sibbel Travel award to present her research at ISSCR and received a leadership award from the BME Department.
Punn Augsornworawat received a BME Department travel award to present his research at BMES.
Maddy Goedegebuure received an Amgen Scholarship to continue performing important work on maturation of stem cell-derived beta cells this summer.
Anu Sharma has been accepted to the Technion-Cornell Master’s Degree in Health Tech program.
Arvind Srivatsava has been accepted to the University of Rochester to pursue at PhD.
Unfortunate for us, this means that this is Anu and Arvind’s last week in the lab. We wish them well in their new degree programs
Acquisition of Dynamic Function in Human Stem Cell-Derived β Cells
 January 17, 2019
January 17, 2019
This is Leo’s first research article! Congratulations to him and the rest of the team!
Methods and compositions for increased safety of stem cell-derived populations
January 15, 2019
Dr. Millman issued a new patent entitled “Methods and compositions for increased safety of stem cell-derived populations,” patent number 10,179,901.



































































