News prior to 2019 archived
Millman Lab at the Dream Gala St. Louis
April 18, 2024
Members of the Millman Lab and their families attended the 2024 JDRF Dream Gala St. Louis on Saturday. The gala is a fundraising event for community and corporate leaders in the T1D community benefiting the JDRF Kansas & Missouri chapter.
Clinical Trial Featuring Millman Lab Research Gets Update
April 15, 2024
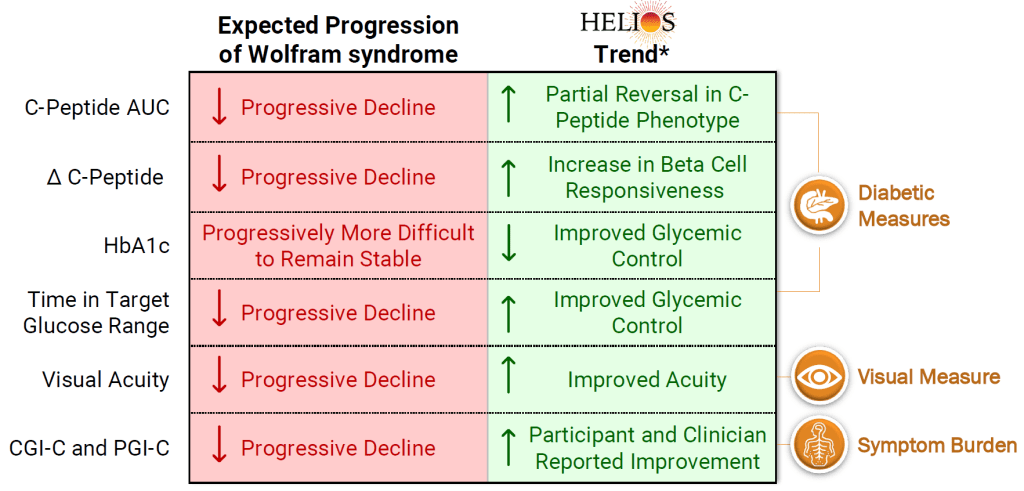
Last week, Amylyx Pharmaceuticals released interim data from the ongoing HELIOS clinical trial that features Millman Lab research. The trial is using AMX0035 to improve pancreatic function and glycemic control in Wolfram Syndrome patients. The Millman Lab previously used SC-islets that were made from patients with Wolfram Syndrome to do a preclinical evaluation of this compound. The interim data showed that patients had an increase in C-peptide and beta cells had more rapid responses to glucose challenge, which was unexpected due to disease progression. Additionally, most patients had a decrease in A1c and an increase in time in range. There were other benefits as well reflected in the summary graphic above. Read more about the preclincal study here and the update here.
Dr. Millman Featured On The Sugar Science Podcast
March 5, 2024
Dr. Millman was featured in the “Ask the Expert” segment of The Sugar Science podcast. He discussed using single-cell sequencing to understand the cellular complexity of SC-Islet identity.
Ella and Liam Join the Lab
February 19, 2024
Ella Hanson and Liam Oiknine joined the lab as trainees this month. Ella is in the B.S./M.S. Biomedical Engineering program at WashU and Liam is a Freshman at WashU, studying Computer Science and Biology. Welcome, Ella and Liam!
New Paper in BMC Genomics
January 24, 2024
Mason Schmidt (former postbacc student), Matt Ishahak, Punn Augsornworawat (former Ph.D. student) and Dr. Millman published a paper in BMC Genomics. The publication reveals comparisons of SC-islets, fetal islets, and adult islets using single-cell sequencing datasets to characterize the transcriptional identity of each. The team’s analysis revealed that SC-β cells share a core β-cell transcriptional identity with human adult and fetal β-cells, however SC-β cells possess a unique transcriptional profile. Read the open-access article here.
2023 Holiday Party
Millman Lab Recognized at the COVID-19 Awards
November 28, 2023

Drs. Millman and Hogrebe were recognized at the WashU COVID-19 Awards. The COVID-19 Awards recognize outstanding individuals for their work and contributions in the Department of Medicine during the pandemic.
Dr. Millman was awarded the COVID Star Award and Dr. Hogrebe was awarded the COVID Rising Star Award.
Congratulations, Drs. Millman and Hogrebe!
Millman Lab Receives Green Lab Certification at Green Carpet Awards
 November 21, 2023
November 21, 2023
The Green Carpet Awards are an annual award ceremony honoring WashU labs and offices that certified as Sustainability Champions through the WashU Sustainability office. The Millman Lab was certified at the Gold level for its efforts to conduct research in more sustainable ways. Erika Brown (back right) attended the awards as the lab’s Green Lab representative. Read more about the awards here and the Green Labs program here.
Millman Lab Participates in 2023 Diabetes Day Symposium
The WashU Diabetes Research Center hosts an annual diabetes symposium in honor of World Diabetes Day, which falls on November 14. The event brings together DRC scientists and their trainees to showcase the latest developments in diabetes research. During the symposium, Dr. Lori Sussel, Research Director of the Barbara Davis Center for Diabetes at the University of Colorado, delivered a lecture. Danny Veronese Paniagua, Ed Castro-Sanchez, Kameron Bradley, Marlie Maestas, and Matt Ishahak presented posters throughout the day-long event.
Dr. Millman Speaks at 50th Kilo Diabetes Symposium

Dr. Millman spoke at the 50th annual Kilo Diabetes Symposium on Saturday. He gave a talk about Cell Therapy for T1D and participated in a panel discussion on T1D Prevention and Reversal.
The Kilo Diabetes Symposium is a national forum for leaders in the field to educate the medical community about research findings and good clinical practice in diabetes, endocrinology, and cardiovascular diseases.
Millman Lab at 2023 JDRF One Walk
Several members of the Millman Lab and their families came out to the 2023 JDRF One Walk. The one-mile walk is a fundraiser for diabetes research and a way for those living with T1D and their friends and families to come together and support the fight for a cure. It was a beautiful day to spend together and support our friends at JDRF!
Matt and Kameron Present at BMES 2023
Dr. Matt Ishahak and Kameron Bradley spent last week at the 2023 Biomedical Engineering Society (BMES) Annual Meeting in Seattle, WA. Kameron presented a poster on his hypoxia work and Matt gave a talk on the lab’s HIRN catalyst project. Matt also received an award from the Engineering Endocrine Tissue Special Session.
Millman Lab at Environmental Justice Day of Action

Dr. Millman, Charlotte Millman, Alexa Wagner, Matt Ishahak, and Erika Brown participated in a neighborhood cleanup in O’Fallon Park in North City St. Louis on Saturday. The cleanup was part of St. Louis Environmental Justice Days of Action, an initiative to address environmental justice issues such as illegal dumping and food apartheid, which is led by earthday365 and North Newstead Association. It was a great day to help out the local community!
Alexa Joins the Lab

Alexa Wagner joins the lab as a Research Assistant. Welcome, Alexa!
Dr. Ishahak Wins Award

Dr. Matt Ishahak received the Distinguished Postdoctoral Scholars award from the Center of Regenerative Medicine. This program is designed to develop highly trained individuals who can produce impactful research in regenerative medicine through an interdisciplinary training plan. Congratulations, Matt!
Drs. Millman and Ishahak Attend HIRN Annual Meeting
Dr. Millman and Dr. Matt Ishahak attended the Human Islet Research Network (HIRN) annual meeting last week. They both presented at the meeting and Dr. Millman participated in the hypoimmune islet panel.
Millman Lab at Environmental Justice Day of Action

Dr. Millman, Charlotte Millman, Ed Castro-Sanchez, Marlie Maestas, and Erika Brown participated in a neighborhood cleanup in Jennings, MO on Saturday. The cleanup was part of St. Louis Environmental Justice Days of Action, an initiative to address environmental justice issues such as illegal dumping and food apartheid, which is led by earthday365. It was a great day to spend some time together and do some good for the community!
Cam’s Last Day

The Millman Lab said goodbye to Cameorn Banks on Friday with lunch from Lona’s Lil Cafe. Cam is heading to a Ph.D. program at the University of Minnesota in the fall where he will study within the Microbiology, Immunology, and Cancer Biology (MICaB) program. Good luck, Cam; we will miss you!
Veronica’s Last Day

Veronica Lee had her last day of her summer research period in the lab on Wednesday. She will continue her undergraduate study at WashU. Good luck, Veronica!
Diana Promoted

Diana Hernandez has been promoted to Senior Research Technician. Congratulations, Diana!
Camryn Gives Talk at uSTAR Closing Symposium

Camryn Moore spoke on her research in the Millman Lab at the uSTAR Summer Scholars Program Closing Symposium. The talk was entitled “CRISPR Validation and Functional Analysis of Hypoxic and Normoxic Stem Cell-Derived Islets.” Great job, Camryn!
JDRF Kids Visit Millman Lab
The Millman Lab had a recent visit from the local chapter of JDRF, where we had the pleasure of hosting several families with children affected by T1D. Guests were given a tour of the lab with talks by Millman Lab members. A reception followed with pipette racing, a sink or float experiment, and a photo booth. Thank you to Millman Lab volunteers, JDRF, and the families!
Lab Certifies Gold With WashU Green Labs

The Millman Lab has been certified gold with WashU’s Green Labs Program. Read More about some of our sustainability efforts here!
Mason Farewell Lunch

The Millman Lab said goodbye to Mason Schmidt on Monday with lunch from Gioia’s Deli. Mason is heading to a Ph.D. program at Duke in the fall where he will study within the Developmental and Stem Cell Biology program. Good luck, Mason; we will miss you!
Dr. Millman Speaks at 2023 Endocrine Society
Millman Lab Presents at ISSCR

Kameron Bradley, Danny Veronese Paniagua, Matt Ishahak, Diana Hernandez, and Marlie Maestas attended the 2023 International Society for Stem Cell Research (ISSCR) meeting in Boston, MA. They presented posters on the latest research from the lab.
Millman Lab Featured in WashU News

The Millman Lab’s latest publication in Nature Cell Biology was featured in a press release by WUSM News. The article explains the study, which was on creating improved stem cell-derived beta cells through machine learning and computational methods. Read the article here.
Camryn and Veronica Join the Lab
Camryn Moore and Veronica Lee both join the lab as undergraduate trainees. Camryn is with the WashU U-Star Summer Scholar Program and Veronica is with the WashU Amgen Scholars Program. They will be mentored by Kameron Bradley and Marlie Maestas respectively. Welcome, Camryn and Veronica!
Allison Joins the Lab

Allison Kelley joins the lab as a Research Technician. Welcome, Allison!
New Paper in Nature Cell Biology

Link to the open access article here.
Diana Graduates
Ed Joins the Lab

New Paper in Nature Biotechnology

Link to the open access article here.
Lab Participates in Park Cleanup

Members of the Millman Lab and their families participated in a cleanup of O’Fallon Park in St. Louis. The cleanup was part of St. Louis Environmental Justice Days of Action, an initiative to address environmental justice issues such as illegal dumping and food apartheid. It was a great day to spend some time together and do some good for the community.
New Paper in Cell Stem Cell

Link to the open access article here.
Millman Lab Lunch

Dr. Millman Study Featured on JDRF News

Aining Passes Masters Thesis Defense

Chandler Accepts Position at Bayer

Mira Joins the Lab

Mason Awarded NSF Fellowship

Punn Defends Thesis

Matt Accepts Position On WashU Council

Danny Receives Award

Cameron Accepted Into Graduate School

JDRF Visit









Nathaniel Hogrebe Promoted

Lab Holiday Party

Nathaniel Hogrebe Presented at GESC Symposium

Erika joins the Millman lab

Punn Augsornworawat receives award on Diabetes day

Daniel Veronese-Paniagua at SACNAS NDiSTEM

Punn Augsornworawat, Matthew Ishahak, and Aining Fan at BMES 2022


Punn Augsornworawat at TERMIS-AP 2022 South Korea

New paper on Advanced Materials Technologies

New paper on JCI Insight

Millmab lab at DBBS Retreat



Matthew Ishahak presented at UNITE

Danny is part of E4B

Jeffrey Millman receives HIRN grant

Punn Augsornworawat featured on The Scientist


Jeffrey Millman named Mallinckrodt Scholar

Dr. Millman on AP news
July 19, 2022
![]()
Jeffrey Millman, PhD. was recently interviewed by the Associated Press on growing stem cells in space. This topic covers interesting areas on whether or not stem cells can grow in the zero gravity condition. Check out the this link here.
Matthew awarded by NIH
 Matthew Ishahak was selected for the NIH National Research Service Award (NRSA) postdoctoral fellowship on the Diabetes and Related Metabolic Diseases Training grant in the Division of Endocrinology at Washington University School of Medicine (5 T32 DK007120)
Matthew Ishahak was selected for the NIH National Research Service Award (NRSA) postdoctoral fellowship on the Diabetes and Related Metabolic Diseases Training grant in the Division of Endocrinology at Washington University School of Medicine (5 T32 DK007120)Millman lab team presents at ISSCR 2022
June 18, 2022

Mason joins dkNET Summer of Data Student Program
June 2, 2022

Mason Schmidt, our lab’s postgraduate researcher, joins the 2022 dkNET Summer of Data Student Program and is awarded a scholarship for the program. Congratulations!
Julia Miller graduates from Washu!
June 1, 2022

Julia Miller graduated with a Bachelor of science in biomedical engineering this summer. She has been actively working with the Millman Lab on engineering Stem cells to produce pancreatic islets. Congratulations!
Augsornworawat wins BME research award
May 11, 2022

Punn Augsornworawat received the Biomedical Engineering (BME) Outstanding Research Award on May 2, 2022. His works are focused on developing methodologies to generate pancreatic islets from stem cells and utilizing single-cell technologies to study islet development and β-cell maturation.
Maggie joins the Lab!
April 4, 2022

We are proud to announce that Maggie Bui, MSTP student, will be joining our lab to work on her PhD in islet development and biology.
New publication on Stem Cells Translational Medicine
March 21, 2022
We are proud to share our new publication “Differential Function and Maturation of Human Stem Cell-Derived Islets After Transplantation”. This study explores different transplantation strategies for SC-islets in diabetic mice. Link

“A Possible Cure for Diabetes”
February 1, 2022
Dr. Millman is featured in Eureka’s Podcast “A Possible Cure for Diabetes”. Check out on our research highlights and current progress in Diabetes cure. Link 
Millman lab welcomes new members!
Janurary 20, 2022
We are excited to welcome 2 new members to the Millman Lab. Kameron joined us as a doctoral student from the department of biomedical engineering. Aining, from the department of biomedical engineering, joined us a Masters student. Welcome!


Dr. Millman was interviewed for an article in IEEE Pulse
November 9, 2021
Dr. Millman was interviewed for an article in IEEE Pulse about the promises and challenges of stem cells for diabetes cell replacement therapy. Check out the article here!
Julia Receives Future Leaders in Chemical Engineering Award
November 8, 2021
We are proud to see Julia Miller, an Undergraduate student in Biomedical Engineering who has been working with us, receiving a Future Leaders in Chemical Engineering award! Julia’s work is on genetic engineering of Stem cell derived Islets. Congratulations Julia!

Dr. Est joins Millman lab!
October 18, 2021
Millman lab welcomes new Post-doctoral fellow Dr. Chandler Est! Chandler will be studying Islet differentiations using genetic engineering tools and developing new approaches to investigate diabetes.

Millman lab presents at BMES 2021 in Orlando
October 15, 2021
Last week, Punn Augsornworawat, Matthew Ishahak, and Julia Miller gave oral presentations at the 2021 BMES conference in Orlando, Florida. Each presented their Stem cell and Diabetes research work.

Dr. Millman is featured on an interview with Nature
September 29, 2021
Nature Publishing Group interviewed Dr. Millman on the feasibility and current efforts in scaling and manufacturing cells for cell replacement therapies. The interview is featured in “Stem-cell start-ups seek to crack the mass-production problem”
Matthew receives NIH LRP award
August 6, 2021
Big congratulations to Dr. Ishahak for receiving the NIH LRP award from the National institute of Diabetes and Digestive and kidney diseases (NIDDK).

Published in Nature Protocols!
August 3, 2021
Our strategy for making islets from ES/iPS cells is now out in Nature Protocols. In this article, Co-first authors Hogrebe, Maxwell, and Augsornworawat illustrate making islets from patient iPSCs (T1D, T2D, MODY, Wolfram Syndrome). We hope that this is a useful resource for other labs.

Congratulations Dr. Leo!
July 27, 2021
Leonardo defended his PhD Thesis on the functional development and maturation of SC-Beta cells. Huge congrats to Dr. Velazco-Cruz!

New Paper on Oxygen Effect on Stem Cell Differentitations
July 12, 2021
Dr. Millman publishes new research article on the effects of physiological oxygen on teratoma formation in Stem cell differentiations
Free access to the article can be found found here: Link
Review Paper on Islet Encapsulation Published
June 22, 2021
Dr. Millman publishes a review article on designing encapsulation devices using SC-Islets for T1D treatment.
New Publication in Science Translational Medicine
June 2, 2021
A team of researchers led by diabetes specialists and biomedical engineers at Washington University School of Medicine in St. Louis and Cornell University has demonstrated that, using a miniscule device, they can implant insulin-secreting cells into diabetic mice. Once implanted, the cells secrete insulin in response to blood sugar, reversing diabetes without requiring drugs to suppress the immune system.

The findings are published June 2 in the journal Science Translational Medicine.
*Portions of this originally published by Washington University School of Medicine
Dr. Millman receives tenure!
May 14, 2021
Dr. Jeffrey Millman has been promoted to Associated Professor and granted tenure effective June 1!

Michelle joins Duke University for the MD Program
May 7, 2021
Michelle joined the Millman lab in 2017 and made huge contributions to our work in Diabetes Research. She will now be joining the MD program at Duke University. We bid her farewell and wish her great successes in her future endeavors!
Millman lab members win company pitch competition
April 27, 2021
Millmab Lab members, Leo, Punn, Kristina and Dr. Millman, led Salentra Biosciences is awarded $7500 in funding from the Skandalaris Venture competition.

New review on IPS derived Beta cell applications
April 20, 2021
Dr. Maxwell and Dr. Millman publishes a new review article on the applications of IPS derived Beta cells!

Marlie joins the Millman Lab!
January 29, 2021
Marlie Maestas joins the Millman Lab as a doctoral student from the DBBS program. Welcome Marlie!

Millman Lab Welcomes new members!
February 14, 2021
The Millman Lab is proud to have additional members join our team. Sarah Gale joins us as our senior lab manager. Diana and Cameron are here to join us as Lab technicians. The two will be conducting research in optimizing and expanding in vitro Islet generation for diabetes cell replacement therapy.



Dr. Millman Received CMBE Award
January 2, 2021
Dr. Jeffrey Millman was named and awarded the 2021 Cellular and Molecular Bioengineering (CMBE) Rising Star by BMES. Congratulations!

Congratulations Dr. Maxwell
December 7, 2020
Big congrats to Millman lab Doctoral student, Kristina Maxwell for successfully defending her thesis entitled “Autologous Stem Cell-Derived β Cells for Diabetes Cell Replacement Therapy”. Her work has provided significant contributions to the diabetes cell replacement therapy field. Congratulations Dr. Maxwell!

Millman Lab participates JDRF One Walk
October 31, 2020
Millman Lab team participates this year’s JDRF One Walk fundraising event. The “Beta Squad” team raised funds and enjoyed the St. Louis’s forest park stroll to support research in curing Type I Diabetes.

Millman Lab at BMES 2020
October 14, 2020
Millman Lab team, Punn Augsornworawat, Kristina Maxwell and Dr. Jeffrey Millman himself will be giving talks at the 2020 BMES Annual meeting. Please check out their talks and feel free to drop by for the live Q&A sessions.
Kristina Maxwell – Stem Cell Engineering I October 15 3PM-4PM
Jeffrey Millman – Stem Cell Engineering II October 15 9AM-10AM
Punn Augsornworawat – Stem Cell Engineering II October 15 9AM-10AM



Dr. Millman featured on Podcast
September 19, 2020
Dr. Jeffrey Millman spoke at a podcast on the development and advancements of Stem cells for treating type I diabetes.

Check it out from these links:
Apple Podcasts bit.ly/JBPAPod
Spotify bit.ly/JBPspot
Amazon Music bit.ly/JBPAmazonMusic
Online bit.ly/JuiceboxPod
Leo is awarded NIH F31
August 26, 2020
 Congratulations to Millman Lab Doctoral student Leonardo Velazco-Cruz for getting the prestigious NIH F31 Fellowship! His excellent achievements have proven him worthy of this award. The team is proud of his accomplishments.
Congratulations to Millman Lab Doctoral student Leonardo Velazco-Cruz for getting the prestigious NIH F31 Fellowship! His excellent achievements have proven him worthy of this award. The team is proud of his accomplishments.
August 25, 2020
 Millman lab’s recent paper, Single-Cell Transcriptome Profiling Reveals β Cell Maturation in Stem Cell-Derived Islets after Transplantation, is featured on the Cell Reports Journal cover! Check out the released journal issue on the link below.
Millman lab’s recent paper, Single-Cell Transcriptome Profiling Reveals β Cell Maturation in Stem Cell-Derived Islets after Transplantation, is featured on the Cell Reports Journal cover! Check out the released journal issue on the link below.
August 25, 2020

Stem Cell-Derived Islets Now Resemble Human Islets
Researchers at Washington University have been generating insulin-producing islets in the laboratory from human stem cells. This approach promises a virtually unlimited supply of replacement insulin-producing tissues to functionally cure patients with diabetes. However, cell-derived islets are immature compared to islets from the body, lacking the expression of genes associated with maturation.
Here, a team of researchers from the laboratory of Dr. Jeffrey Millman at Washington University in St. Louis have discovered that these stem cell-derived islets are capable of maturing after transplantation to resemble real islets more closely from humans. The team, with co-first authors Punn Augsornworawat and Kristina Maxwell, accomplished this feat by developing a new methodology to isolate transplanted cells and performed single-cell RNA sequencing to catalogue these changes in individual cells.
The mice used in the experiments had severe diabetes. After several months, these mice were completely cured of diabetes, with the transplanted stem cell derived islets providing robust insulin release and blood glucose regulation, equivalent to healthy mice.
These long-term improvements in function are associated with increased expression of mature genes, such as MAFA and G6PC2. In addition, the team also identified other mature markers including FAM159B, and MT1X, to robustly confirm maturation and found that secretion of the peptide IAPP to be a detectable biomarker for maturation more robustly.
These findings highlight the similarities between lab-grown islets after transplantation and native islets from the body and provides a new technological pipeline allowing for more rigorous study of cellular maturation of differentiated cells from stem cells. The research article was published on August 25, 2020 in the journal of Cell Reports.
This study was supported by the National Institutes of Health (5R01DK114233, T32DK108742, P30CA91842, UL1TR000448), a JDRF Career Development Award (5-CDA-2017-391-A-N), Washington University-Centene Personalized Medicine Initiative, David and Deborah Winston Fellowship in Diabetes Research, and joint funds from Washington University (Departments of Surgery, Medicine, and Pediatrics), Mid-America Transplant Services, and The Foundation for Barnes-Jewish Hospital.
New review article on single-cell technologies
August 4, 2020

Punn Augsornworawat and Dr. Millman published a review article on Single-cell RNA sequencing to investigate pancreas development and diabetes.
Leo and Maddy published new article
July 9, 2020


Leonardo Velazco-Cruz and Madeleine Goedegebuure published a review article on β cell development and functional maturation. Congratulations!
Millman lab PhD students win travel awards
Matt receives Rita Levi-Montalcini Postdoctoral Fellowship
July 1, 2020
Congratulations to our Postdoctoral Fellow, Dr. Matthew Ishahak, for receiving the first Rita Levi-Montalcini Postdoctoral Fellowship in Regenerative Medicine!

Millman lab welcomes new members!
June 15, 2020
We are pleased to welcome Erica Marquez, and the Rita Levi-Montalcini postdoctoral fellow Dr. Matthew Ishahak to join our team. Dr. Ishahak will be using his microfluidic expertise to design devices to model and study the behaviors of stem cell-derived islets. Ms. Marquez will explore strategies to genetically engineer stem cell-derived islets for studying diabetes. We look forward to their future contributions to the diabetes therapy field.
New publication in Cell Reports
May 26, 2020

Congratulations to Leo, Maddy, and the rest of the team for their new publication in Cell Reports! In it, they discovered that the transcription factor SIX2 is essential for the functional maturation of stem cell-derived beta cells. This is important as our team continues to engineer these cells for diabetes cell replacement therapy.
Generation of insulin-secreting β cells in vitro is a promising approach for diabetes cell therapy. Human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs) are differentiated to β cells (SC-β cells) and mature to undergo glucose-stimulated insulin secretion, but molecular regulation of this defining β cell phenotype is unknown. Here, we show that maturation of SC-β cells is regulated by the transcription factor SIX2. Knockdown (KD) or knockout (KO) of SIX2 in SC-β cells drastically limits glucose-stimulated insulin secretion in both static and dynamic assays, along with the upstream processes of cytoplasmic calcium flux and mitochondrial respiration. Furthermore, SIX2 regulates the expression of genes associated with these key β cell processes, and its expression is restricted to endocrine cells. Our results demonstrate that expression of SIX2 influences the generation of human SC-β cells in vitro.
Congratulations to Maddy for graduating!
May 22, 2020
 Maddy will be moving on to do a PhD at Northwestern. We will miss you!
Maddy will be moving on to do a PhD at Northwestern. We will miss you!
Diabetes reversed in mice with genetically edited stem cells derived from patients – new publication in Science Translational Medicine
April 22, 2020
Using induced pluripotent stem cells produced from the skin of a patient with a rare, genetic form of insulin-dependent diabetes called Wolfram syndrome, researchers transformed the human stem cells into insulin-producing cells and used the gene-editing tool CRISPR-Cas9 to correct a genetic defect that had caused the syndrome. They then implanted the cells into lab mice and cured the unrelenting diabetes in those mice.
The findings, from researchers at Washington University School of Medicine in St. Louis, suggest the CRISPR-Cas9 technique may hold promise as a treatment for diabetes, particularly the forms caused by a single gene mutation, and it also may be useful one day in some patients with the more common forms of diabetes, such as type 1 and type 2.
The study is published online April 22 in the journal Science Translational Medicine.
Patients with Wolfram syndrome develop diabetes during childhood or adolescence and quickly require insulin-replacement therapy, requiring insulin injections multiple times each day. Most go on to develop problems with vision and balance, as well as other issues, and in many patients, the syndrome contributes to an early death.
“This is the first time CRISPR has been used to fix a patient’s diabetes-causing genetic defect and successfully reverse diabetes,” said co-senior investigator Jeffrey R. Millman, PhD, an assistant professor of medicine and of biomedical engineering at Washington University. “For this study, we used cells from a patient with Wolfram syndrome because, conceptually, we knew it would be easier to correct a defect caused by a single gene. But we see this as a stepping stone toward applying gene therapy to a broader population of patients with diabetes.”
Wolfram syndrome is caused by mutations to a single gene, providing the researchers an opportunity to determine whether combining stem cell technology with CRISPR to correct the genetic error also might correct the diabetes caused by the mutation.
A few years ago, Millman and his colleagues discovered how to convert human stem cells into pancreatic beta cells. When such cells encounter blood sugar, they secrete insulin. Recently, those same researchers developed a new technique to more efficiently convert human stem cells into beta cells that are considerably better at controlling blood sugar.
In this study, they took the additional steps of deriving these cells from patients and using the CRISPR-Cas9 gene-editing tool on those cells to correct a mutation to the gene that causes Wolfram syndrome (WFS1). Then, the researchers compared the gene-edited cells to insulin-secreting beta cells from the same batch of stem cells that had not undergone editing with CRISPR.
In the test tube and in mice with a severe form of diabetes, the newly grown beta cells that were edited with CRISPR more efficiently secreted insulin in response to glucose. Diabetes disappeared quickly in mice with the CRISPR-edited cells implanted beneath the skin, and the animals’ blood sugar levels remained in normal range for the entire six months they were monitored. Animals receiving unedited beta cells remained diabetic. Their newly implanted beta cells could produce insulin, just not enough to reverse their diabetes.
“We basically were able to use these cells to cure the problem, making normal beta cells by correcting this mutation,” said co-senior investigator Fumihiko Urano, MD, PhD, the Samuel E. Schechter Professor of Medicine and a professor of pathology and immunology. “It’s a proof of concept demonstrating that correcting gene defects that cause or contribute to diabetes — in this case, in the Wolfram syndrome gene — we can make beta cells that more effectively control blood sugar. It’s also possible that by correcting the genetic defects in these cells, we may correct other problems Wolfram syndrome patients experience, such as visual impairment and neurodegeneration.”
In the future, using CRISPR to correct certain mutations in beta cells may help patients whose diabetes is the result of multiple genetic and environmental factors, such as type 1, caused by an autoimmune process that destroys beta cells, and type 2, which is closely linked to obesity and a systemic process called insulin resistance.
“We’re excited about the fact that we were able to combine these two technologies — growing beta cells from induced pluripotent stem cells and using CRISPR to correct genetic defects,” Millman said. “In fact, we found that corrected beta cells were indistinguishable from beta cells made from the stem cells of healthy people without diabetes.”
Moving forward, the process of making beta cells from stem cells should get easier, the researchers said. For example, the scientists have developed less intrusive methods, making induced pluripotent stem cells from blood — and they are working on developing stem cells from urine samples.
“In the future,” Urano said, “we may be able to take a few milliliters of urine from a patient, make stem cells that we then can grow into beta cells, correct mutations in those cells with CRISPR, transplant them back into the patient, and cure their diabetes in our clinic. Genetic testing in patients with diabetes will guide us to identify genes that should be corrected, which will lead to a personalized regenerative gene therapy.”
[portions of this was taken from the WashU press release by Jim Dryden]
New patent issued
April 17, 2020
The team just had a new patent issued for our invention of using 3D printing to make a transplantable scaffold for stem cell-derived islets.
Jeffrey R. Millman and Jiwon Song. 3D-printed scaffold device for cell transplantation. 10,597,639. Issued March 24, 2020
Danny awarded fellowship from the NSF

March 29, 2020
Danny has received a NSF Graduate Research Fellowship Program (GRFP) fellowship. This award “recognizes and supports outstanding graduate students in NSF-supported science, technology, engineering, and mathematics disciplines who are pursuing research-based Master’s and doctoral degrees at accredited United States institutions”, according the NSF website.
Danny joins the lab!

March 23, 2020
We are excited to have Danny join the lab as a PhD student in the DBBS program. He will be studying the molecular mechanisms that control cellular fate and health.
New recent fellowships!


February 26, 2020
Congratulations to Dr. Nathaniel Hogrebe and Punn Augsornworawat for receiving the JDRF Advanced Postdoctoral Fellowship and David and Deborah Winston Fellowship in Diabetes Research Award, respectively!
Diabetes in mice cured rapidly using human stem cell strategy

February 24, 2020
Researchers in the Millman lab have converted human stem cells into insulin-producing cells and demonstrated in mice infused with such cells that blood sugar levels can be controlled and diabetes functionally cured for nine months.
The findings, from researchers at Washington University School of Medicine in St. Louis, are published online Feb. 24 in the journal Nature Biotechnology.
“These mice had very severe diabetes with blood sugar readings of more than 500 milligrams per deciliter of blood — levels that could be fatal for a person — and when we gave the mice the insulin-secreting cells, within two weeks their blood glucose levels had returned to normal and stayed that way for many months,” said principal investigator Jeffrey R. Millman, PhD, an assistant professor of medicine and of biomedical engineering at Washington University.
Several years ago, the same researchers discovered how to convert human stem cells into pancreatic beta cells that make insulin. When such cells encounter blood sugar, they secrete insulin. Still, previous work has had its limitations and had not effectively controlled diabetes in mice.
Now, the researchers have shown a new technique they developed can more efficiently convert human stem cells into insulin-producing cells that more effectively control blood sugar.
“A common problem when you’re trying to transform a human stem cell into an insulin-producing beta cell — or a neuron or a heart cell —is that you also produce other cells that you don’t want,” Millman said. “In the case of beta cells, we might get other types of pancreas cells or liver cells.”
Off-target pancreas and liver cells don’t hurt anything when implanted into a mouse, but they don’t fight diabetes either.
“The more off-target cells you get, the less therapeutically relevant cells you have,” he said. “You need about a billion beta cells to cure a person of diabetes. But if a quarter of the cells you make are actually liver cells or other pancreas cells, instead of needing a billion cells, you’ll need 1.25 billion cells. It makes curing the disease 25% more difficult.”
Using the new technique, Millman’s team found far fewer off-target cells were produced while the beta cells that were made had improved function. The technique targets the cells’ internal scaffolding, called the cytoskeleton. The cytoskeleton is what gives a cell its shape and allows the cell to interact with its surrounding environment, converting physical cues into biochemical signals.
“It’s a completely different approach, fundamentally different in the way we go about it,” he said. “Previously, we would identify various proteins and factors and sprinkle them on the cells to see what would happen. As we have better understood the signals, we’ve been able to make that process less random.”
Understanding that process has allowed Millman’s team to produce more beta cells. Importantly, the new technique works efficiently across stem cells from multiple different sources, greatly expanding the ability of this technique in the study of disease.
“We were able to make more beta cells, and those cells functioned better in the mice, some of which remained cured for more than a year,” Millman said. “
He explained that there still is much to do before this strategy can be used to treat people with diabetes. They will need to test the cells over longer periods of time in larger animal models and work to automate the process to have any hope of producing beta cells that can help the millions of people who currently require insulin injections to control their diabetes. But the research is continuing.
Portions of this is from the WashU press release linked below:
Celebration dinner!

February 4, 2020
The lab celebrated a couple of paper acceptances, a new fellowship, and several positive reviews on new grants and paper. Great way to start the year off!
Welcome Julia!

January 17, 2020
We welcome Julia into the lab as an undergraduate researcher. She is working with Punn on studying islet heterogeneity.
Welcome Shreya!

January 13, 2020
We welcome Shreya into the lab as an undergraduate researcher. She is working with Kristina on in vitro models of type 2 diabetes.
Congratulations to Michelle for graduating this December

January 8, 2020
Congratulations to Michelle for graduating with her B.S. in Biochemistry from WashU this December!
Dr. Millman awarded a Distinguished Young Alumni Award

January 7, 2020
Dr. Millman was honored with a Distinguished Young Alumni Award from the Chemical and Biomolecular Engineering (CBE) Department at North Carolina State University, the highest award that CBE can bestow on alumni. This honor recognizes the achievements and contributions of alumni to their profession and serves as an inspiration for current and future students.
Holiday lab lunch!

December 13, 2019
Many reason for us to celebrate this year. New papers, grants, fellowships, award, and graduation! Such a wonderful team!
Labsgiving!

November 25, 2019
The lab celebrated “Labsgiving” this week. Many things to be grateful for!
Madeleine Goedegeburre named a Future Leader in Chemical Engineering

October 23, 2019
Maddy was chosen as a Future Leader in Chemical Engineering from North Carolina State University based on her research in the lab engineering stem cells for the study and treatment of diabetes. Congratulations!
Millman lab presents at this the annual BMES meeting

October 17, 2019
Punn and Maddy presented posters on their work studying differentiation fate and developing screening approaches for diabetes. Also encountered Millman lab alumna Jiwon!
New platform for the assembly of islet organoids from stem cells

August 22, 2019
Co-first authors Punn Augsornworawat and Leonardo Velazco-Cruz published an article entitled “A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells” in Acta Biomaterialia. We also congratulate Punn on his first first-author publication! We hope this platform will enable fabrication of advanced regenerative medicine products for diabetes therapy.
Millman lab presents at Oxford and EASD!


August 5, 2019
Jeff and Leo presented at the EASD-Hagedorn Oxford Workshop on the latest protocol for generating functional beta cells for therapy.
Maddy received an Amgen Foundation Scholarship

July 17, 2019
Maddy received an Amgen Scholarship to continue performing research in the Millman lab with stem cells and genetic engineering – congratulations!
Millman lab presents at the annual ISSCR meeting!

June 26, 2019
Kristina presented at the International Society for Stem Cell Research on her work with differentiated patient-derived stem cells
Arvind Srivatsava defends his M.S. thesis

May 8, 2019
Congratulations to Arvind Srivatsava for defending his Biomedical Engineering Master’s Thesis on the microfluidic assessment of stem cell-derived beta cells!
Many Congratulations!
 May 7, 2019
May 7, 2019
Kristina Maxwell received the Greg Sibbel Travel award to present her research at ISSCR and received a leadership award from the BME Department.
Punn Augsornworawat received a BME Department travel award to present his research at BMES.
Maddy Goedegebuure received an Amgen Scholarship to continue performing important work on maturation of stem cell-derived beta cells this summer.
Anu Sharma has been accepted to the Technion-Cornell Master’s Degree in Health Tech program.
Arvind Srivatsava has been accepted to the University of Rochester to pursue at PhD.
Unfortunate for us, this means that this is Anu and Arvind’s last week in the lab. We wish them well in their new degree programs
Acquisition of Dynamic Function in Human Stem Cell-Derived β Cells
 January 17, 2019
January 17, 2019
This is Leo’s first research article! Congratulations to him and the rest of the team!
Methods and compositions for increased safety of stem cell-derived populations
January 15, 2019
Dr. Millman issued a new patent entitled “Methods and compositions for increased safety of stem cell-derived populations,” patent number 10,179,901.














































