The Sadtler research group uses light to both control the growth of inorganic materials and to probe chemical reactions in single particles. Research projects in our group combine the chemical synthesis of inorganic materials and tailoring light-matter interactions at the nanoscale. The members of our group have backgrounds in chemistry, materials science, and chemical engineering. A critical tool in our lab is fluorescence microscopy at the single-particle and single-molecule levels to understand how heterogeneities in the size, shape, and surface chemistry of nanoscale materials affect their chemical reactivity. Our current research interests focus on 1) understanding how crystal defects and surface structure affect the growth and catalytic activity of semiconductor nanocrystals, 2) understanding the role of solid-state miscibility during chemical transformations in nanoscale crystals, and 3) using light-matter interactions to direct the composition and morphology of inorganic materials.

Solid-state chemistry of nanoscale materials: Chemical transformations are remarkably efficient in nanoscale materials owing to the high surface area and short diffusion lengths necessary to complete the transformation. We are interested in using solid-state reactions to alter the composition of nanoscale materials while preserving their morphological features. This research aims to develop new combinations of nanocrystal shapes and compositions that are useful for optoelectronic devices, such as light-emitting diodes and solar cells.
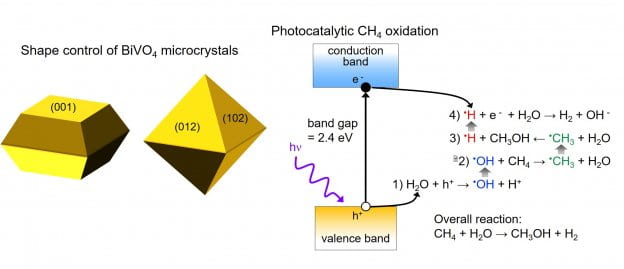
Photocatalysis for fuel generation: The synthesis of chemical fuels from sunlight provides an effective way to store solar energy for use in transportation and to produce commodity chemicals. Semiconductor photocatalysts absorb solar photons and can use the resulting photoexcited charges to drive fuel-forming chemical reactions. Our group is interested in chemical transformations such as the photocatalytic oxidation of methane to methanol and the reduction of carbon dioxide to liquid products. The crystalline facets present at the surface of the semiconductor catalysts dictate the pathways and lifetimes of photogenerated charge carriers. We use colloidal synthesis and electrodeposition to make shape-controlled semiconductor crystals so that we can correlate their nanoscale morphology with photocatalytic activity.
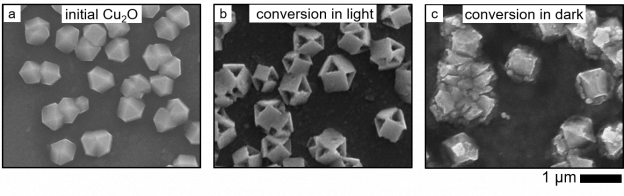
Material growth under external fields. Chemists typically use external parameters such as temperature, pressure, and concentration to direct chemical transformations in molecules and materials. Many classes of materials are also responsive to external stimuli, such as light or electric and magnetic fields. We are creating adaptive inorganic materials that adjust their growth in response to externally controlled fields as a novel route to synthesize three-dimensional nanostructures. Stimuli-driven growth of complex structures can provide new material architectures with enhanced optoelectronic properties for applications in solar energy conversion and photonics.