Research overview
Kristen L. Kroll, Ph.D., Professor, Department of Developmental Biology
Developmental, Regenerative, and Stem Cell Biology Program, Molecular Cell Biology Program, Neuroscience Program
Affiliated with Washington University Intellectual and Developmental Disabilities Research Center (IDDRC), Institute for Clinical and Translational Sciences (ICTS), Hope Center for Neurological Disorders (Hope Center), Center for Regenerative Medicine (CRM).
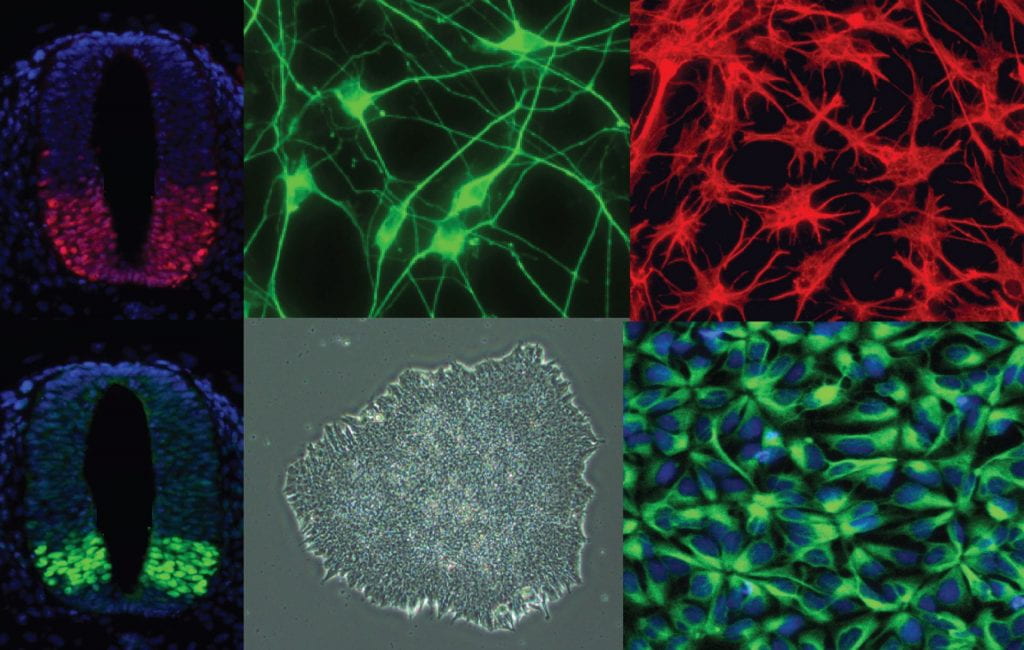
Our research focuses on defining gene regulatory networks that control neural cell specification, neurogenesis, and the generation of specific neuronal cell types. We are interested in understanding how epigenetic regulation modulates these networks and how their dysregulation contributes to neurodevelopmental disorders including epilepsy and autism spectrum disorder and intellectual disability syndromes. This work uses directed differentiation of human pluripotent stem cells (embryonic stem cells and induced pluripotent stem cells), mouse models, and a wide range of cellular, molecular, and genomic approaches, to define roles for transcriptional and epigenetic regulation in shaping developmental transitions.
The lab has three main research interests, outlined below:
- Modeling Neurodevelopmental Disorders in Human Pluripotent Stem Cell Derived-Neurons and Organoids.
- Modeling Human Cortical Interneuron Development and Its Dysregulation in Neurodevelopmental Disorders.
- Gene Regulatory Networks That Regulate Neural Cell Fate Acquisition.
For more information see Research.
Cellular Models Unit for Modeling Neurodevelopmental Disorders
Patient-derived models of neurodevelopmental disorders
My laboratory operates the Cellular Models Unit of the Washington University Intellectual and Developmental Disabilities Research Center (IDDRC). This program represents a unique institutional platform with a mission of performing in-depth studies of intellectual and developmental disability-contributory mechanisms in human cellular models. Our goals are both to link genetic alterations with disease mechanisms and phenotype, and to identify intermediate phenotypes and targets for the development of potential interventions.
PreMIER (Precision Medicine Integrated Experimental Resource)
Harnessing the power of model systems to unravel the complexities of human development and disease
PreMIER (Precision Medicine Integrated Experimental Resource) is a panel of experts that directs clinicians and researchers to appropriate model systems and resources at Washington University, to facilitate progress in our fundamental understanding of biology related to human health and disease. My laboratory coordinates PreMIER’s connections between patients and clinicians and human stem cell and organoid models, while we use these models to assess how gene variants found in patients cause neurodevelopmental disorders.
Radio Interview
Radio Interview over AP article for a station in Melbourne, AU. The interview starts around 1:07:13.
Research Article
Cellular and molecular characterization of multiplex autism in human induced pluripotent stem cell-derived neurons
Lewis, E.M.A., Meganathan, K., Baldridge, D. et al. Cellular and molecular characterization of multiplex autism in human induced pluripotent stem cell-derived neurons. Molecular Autism 10, 51 (2019) doi:10.1186/s13229-019-0306-0
Research Article
Regulation of human cortical interneuron development by the chromatin remodeling protein CHD2
Lewis EMA, Chapman G, Kaushik K, Determan J, Antony I, Meganathan K, Narasimhan M, Gontarz P, Zhang B, Kroll KL. Regulation of human cortical interneuron development by the chromatin remodeling protein CHD2. Sci Rep. 2022 Sep 17;12(1):15636. doi: 10.1038/s41598-022-19654-y.
Research Article
Alterations in neuronal physiology, development, and function associated with a common duplication of chromosome 15 involving CHRNA7
Meganathan K, Prakasam R, Baldridge D, Gontarz P, Zhang B, Urano F, Bonni A, Constantino JN, Kroll KL. Alterations in neuronal physiology, development, and function associated with a common duplication of chromosome 15 involving CHRNA7. bioRxiv. Forthcoming; https://doi.org/10.1101/2020.01.28.922187.
Research Article
Balancing Serendipity and Reproducibility: Pluripotent stem cells as experimental systems for intellectual and developmental disorders
Anderson NC, Chen PF, Meganathan K, Afshar Saber W, Bhattacharyya A, Kroll K, and Sabin M on behalf of the Cross-IDDRC Human Stem Cell Working Group. Balancing Serendipity and Reproducibility: Pluripotent stem cells as experimental systems for intellectual and developmental disorders.Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2021.03.025
Research Article
Epigenetic regulation during human cortical development: seq-ing answers from the brain to the organoid
Emily M. A. Lewisa, Komal Kaushika, Luke A. Sandovala, Irene Antonya, Sabine Dietmanna, Kristen L. Krolla* Epigenetic regulation during human cortical development: seq-ing answers from the brain to the organoid. https://doi.org/10.1016/j.neuint.2021.105039
Research Article
New models to understand the intricacies of neurodevelopmental disorders
Scientia. https://doi.org/10.33548/SCIENTIA663
Review Article
Can the “female protective effect” liability threshold model explain sex differences in autism spectrum disorder?
Dougherty JD, Marrus N, Maloney SE, Yip B, Sandin S, Turner TN, Selmanovic D, Kroll KL, Gutmann DH, Constantino JN, Weiss LA. Can the “female protective effect” liability threshold model explain sex differences in autism spectrum disorder? Neuron. 2022 Oct 19;110(20):3243-3262. doi: 10.1016/j.neuron.2022.06.020.