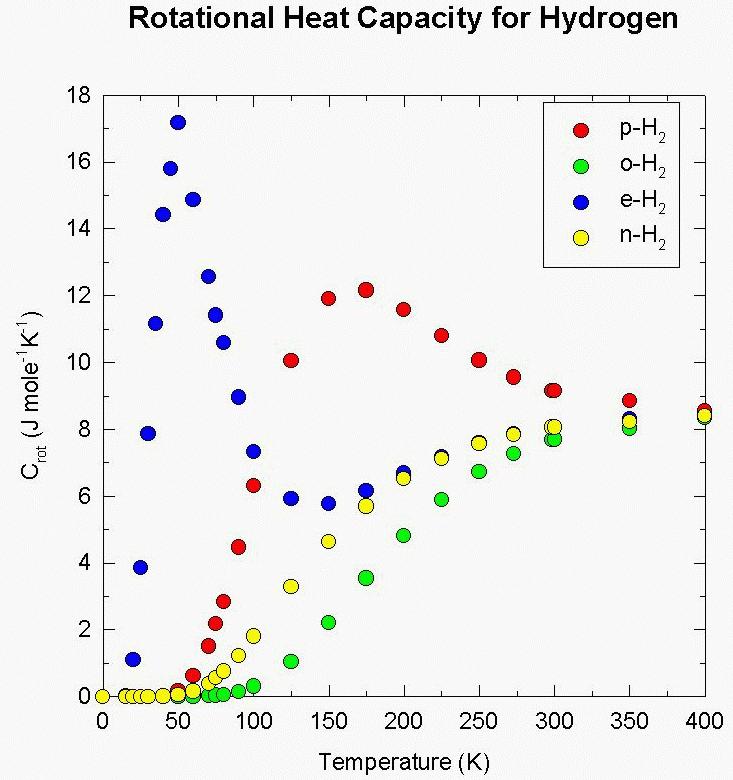
Figure 1. The rotational heat capacity (joules per mole per degree) for hydrogen as a function of temperature. The curves show data for para hydrogen, ortho hydrogen, equilibrium hydrogen, and normal hydrogen. Para hydrogen has antiparallel nuclear spins and even rotational quantum numbers while ortho hydrogen has parallel nuclear spins and odd rotational quantum numbers. Equilibrium hydrogen is the equilibrated o-p hydrogen mixture at any temperature (see Figure 2). Normal hydrogen is a 3:1 mixture of ortho (75%) and para (25%) hydrogen that is the equilibrium composition at high temperatures (i.e., about 298 K and above). From Professor Fegley’s April 1996 presentation to the NASA Outer Planet Science Working Group (OPSWG).