Valosin containing protein (VCP), also named p97 or cdc48, belongs to the ATPase associated with other activities (AAA+) protein family. It has been implicated in multiple cellular processes ranging from cell cycle regulation, DNA replication, organelle biogenesis and protein degradation.
Structure of VCP
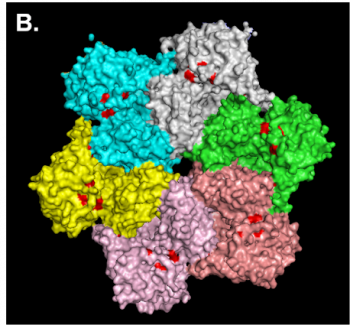
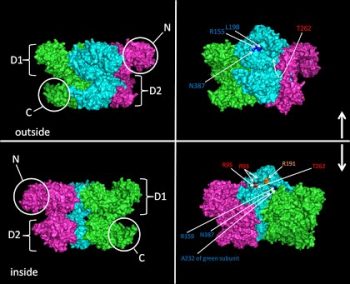
The typical features of a AAA+ protein are one or two tandemly encoded AAA+ protein domains (two in the case of VCP). These domains are essential for ATP binding and hydrolysis. Other features that are less conserved among AAA+ family members include an N- and C-terminal domain. These domains allow for substrate and cofactor selectivity. The active form of most AAA+ proteins is a macromolecular complex consisting of six monomers formed in a ring around a central pore. In the case of VCP both AAA+ protein domains (D1 and D2 domains) sit on top of each other making a stacked or double ring structure. To date 14 unique missense mutations in VCP have been associated with IBMPFD. All mutations reside within the N, linker or D1 domains (Figure A). However, structural analysis demonstrates that these residues reside in a discrete region at the N/D1 domain interface (Figure B and C, mutant residues are marked in red).
Function of VCP
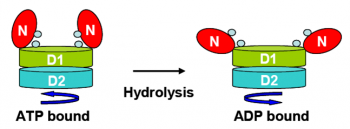
VCP and perhaps all AAA+ proteins, serve as “molecular motors.” During the ATP hydrolysis cycle, VCP undergoes structural changes that alter its affinity for substrates. Because of its “generic motor function”, VCP can participate in many cellular processes depending upon the cofactors which it is associated.
Cofactors of VCP
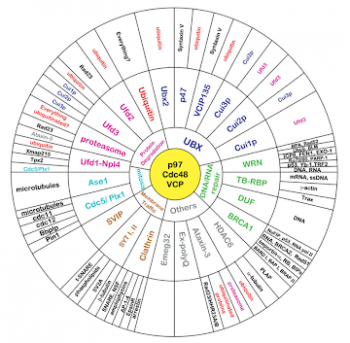
VCP associates with >40 different proteins. These proteins can be grouped into categories consistent with VCP’s function in protein degradation, DNA repair, mitosis, membrane trafficking or organelle biogenesis. For example, VCP’s role in endoplasmic reticulum associated protein degradation (ERAD) requires the co-factors Ufd1/Npl4. In contrast, VCP’s function in golgi biogenesis does not require Ufd1/Npl4 but instead uses p47 as a cofactor.
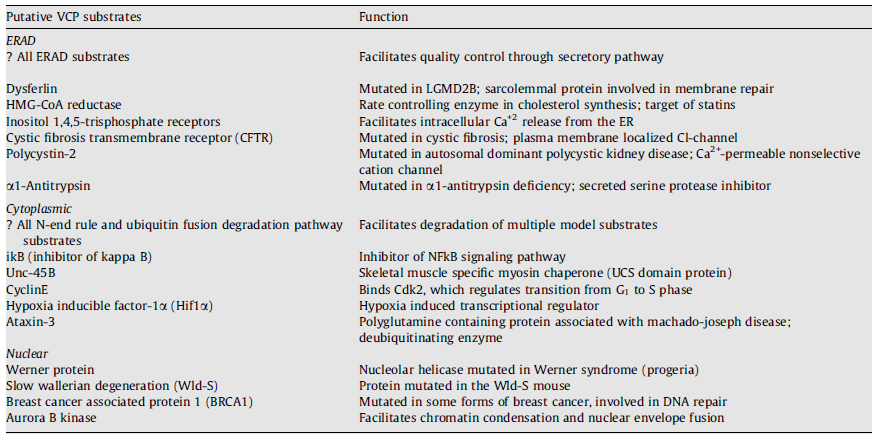
Substrates of VCP
VCP interacts with cofactors and substrates. A VCP substrate, in contrast to a cofactor or adaptor, binds to VCP in an ATP dependent manner. Additionally, the substrate’s degradation or function is then reliant on its interaction with VCP. Several putative VCP substrates exist and are shown below (each with varying supportive evidence). Substrates with the best evidence for VCP dependence on their degradation include most ERAD substrates, Unc-45B, Hif1α and Aurora B kinase.