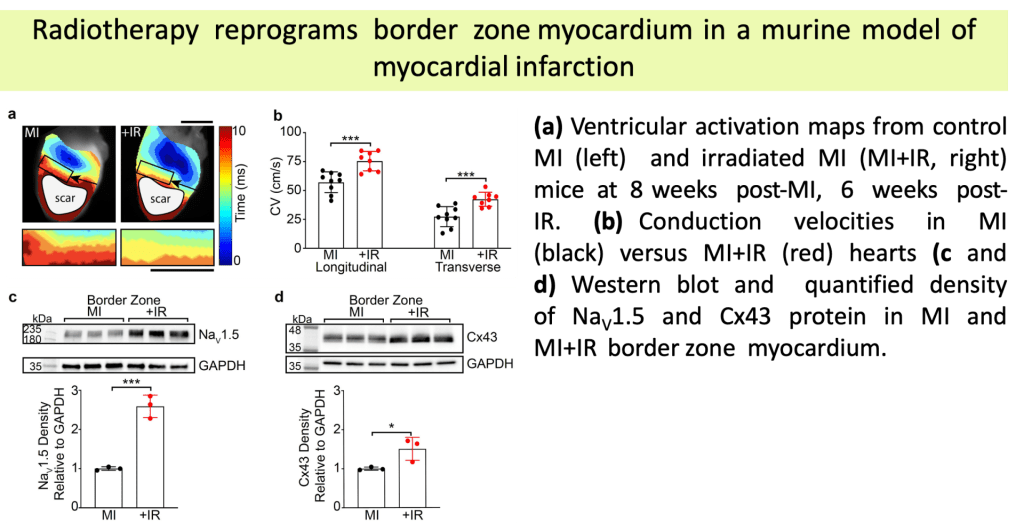
Single Ventricle Defects are a group of rare congenital disorders affecting one of the lower chambers of the heart. The chamber may be smaller, underdeveloped, or missing a valve, resulting in ineffective circulation of oxygenated blood to the body. Approximately 1,000 children are born each year with it. Hypoplastic Left Heart Syndrome (HLHS) is the most frequent form of single ventricle disease. In this case, the heart’s left side — including the aorta, aortic valve, left ventricle, and mitral valve — is underdeveloped.

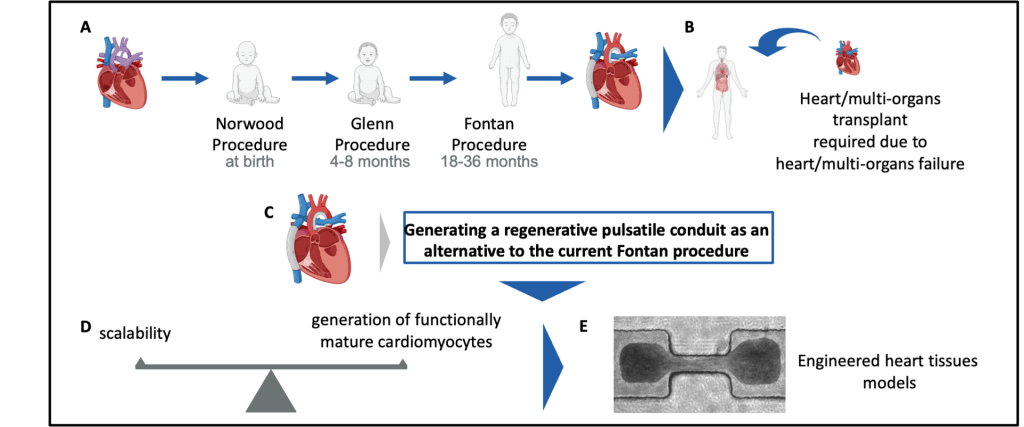
(A) Children born with single ventricle disease undergo three major surgeries at a young age (Norwood, Glenn, and Fontan Procedures)
(B) Despite these surgeries, patients frequently progress to heart or multiorgan (heart/liver/lung) failure at a young age, requiring transplant. Given the limitations of current surgical treatment and the scarcity of organs for transplant, developing new treatments is of great clinical interest.
(C) Our objective is to build a regenerative pulsatile conduit as an alternative to the Fontan procedure.
(D) Generating a functional pulsatile conduit will require balancing scalability and the functional maturity when generating cardiomyocytes.
(E) We utilize various micro- (µEHT) and meso- (dyn-EHT) tissue engineering models to optimize parameters of cardiomyocyte functionality.

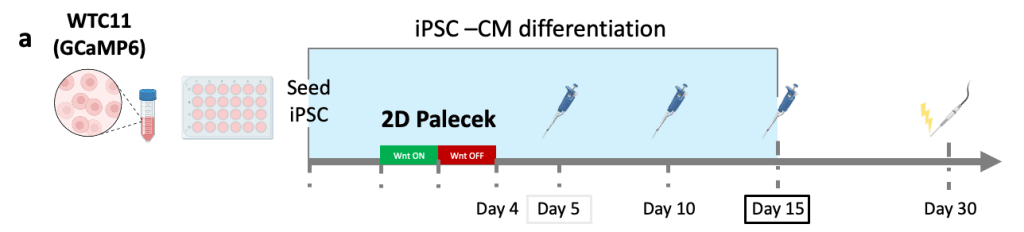
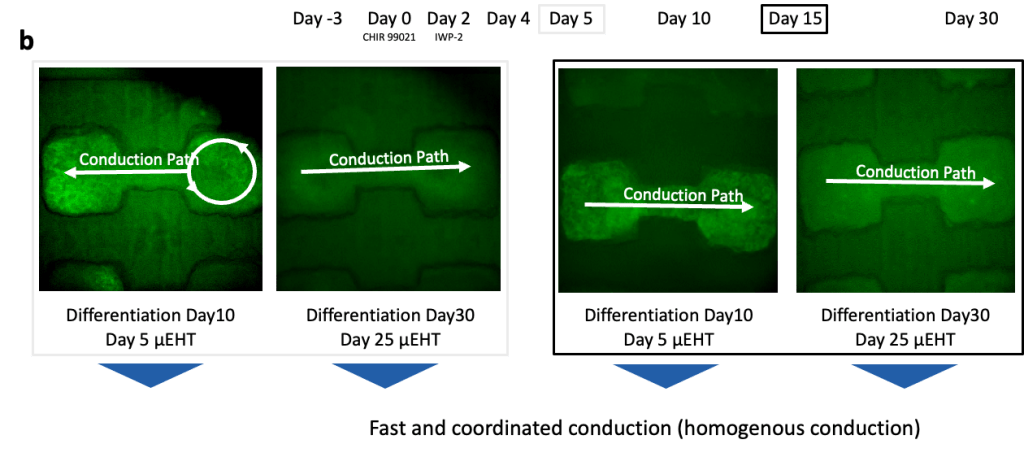
(a) Cardiomyocytes were produced using a 2D Palecek differentiation and loaded in µEHTs at Day 5, Day 10 and Day 15 differentiation.
(b) Calcium GCaMP signal was recorded at different timepoints. At early timepoints (Day 5), we observed disorganized conduction with reentry, but tissues exhibit coordinated conduction at later timepoints, demonstrating appropriate electrophysiologic maturation regardless of cardiomyocyte maturity at the time of seeding.
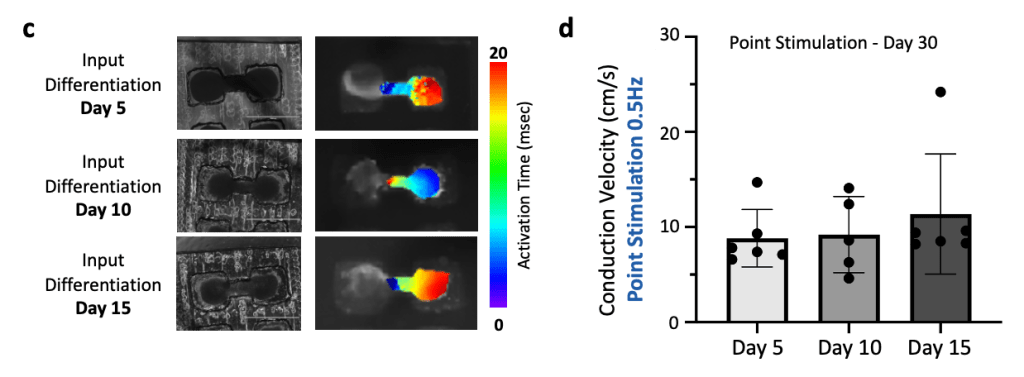
(c) Brightfield microscopy images (left) and associated activation maps (right) for the different input differentiation days (Day 5, 10 and 15).
(d) Conduction velocity analysis shows no significant difference in the conduction velocities.
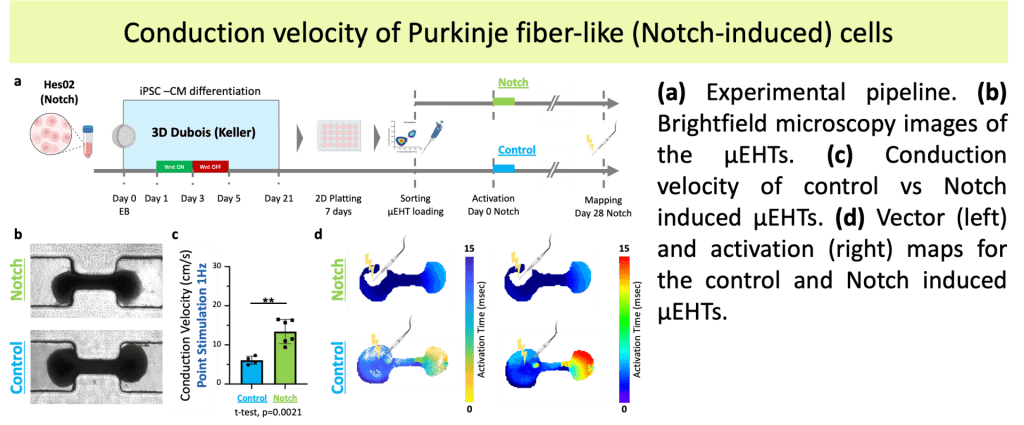






•Established therapies for ventricular tachycardia (VT), a potentially lethal arrhythmia, have limited efficacy and significant morbidity.
•Noninvasive Stereotactic Body Radiation Therapy (SBRT) has recently emerged as a highly efficacious and novel treatment for patients with refractory VT.
•Our group has shown that radiation-induced fibrosis does not occur within the timeframe of observed reductions in VT burden at clinically-efficacious doses.
•Animal models from our lab demonstrated that these doses of radiation durably increase cardiac conduction through upregulation of the cardiac sodium channel (Nav1.5) and gap junction (Cx43), which may favorably reprogram the conduction substrate against re-entry.
•The molecular underpinnings of these mechanisms remain incompletely understood.
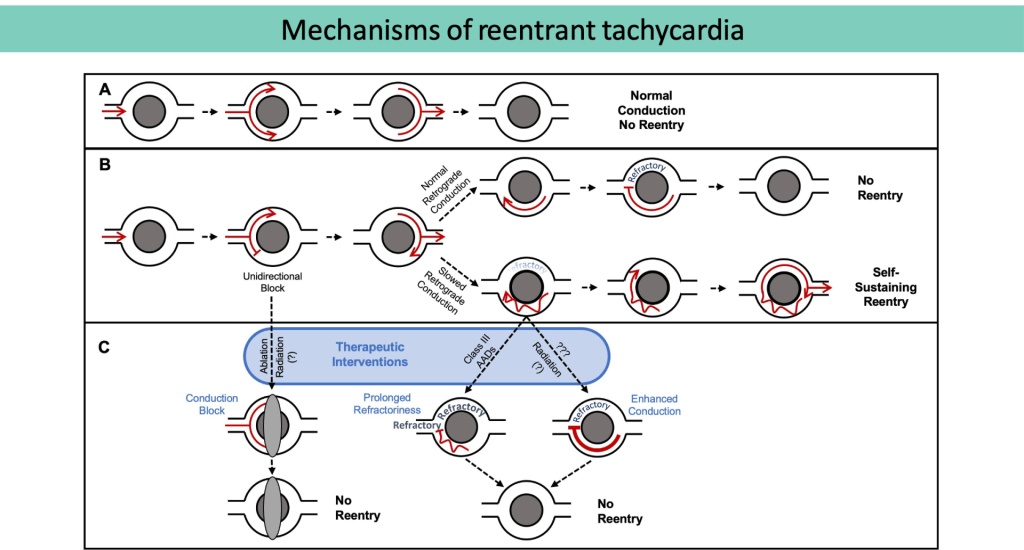
(A) Normal conduction without re-entry.
(B) Unidirectional block, normal (top) versusslowed (bottom) conduction. In the setting of slowed retrograde conduction (bottom), the proximal myocardium is allowed time to repolarize, forming a self-sustaining, re-entrant circuit.
(C) Therapeutic strategies to prevent re-entry include conduction block, prolonging refractoriness, and enhancing conduction.
(Adapted from Zhang DM, et al.Clinical Oncology, 2021)
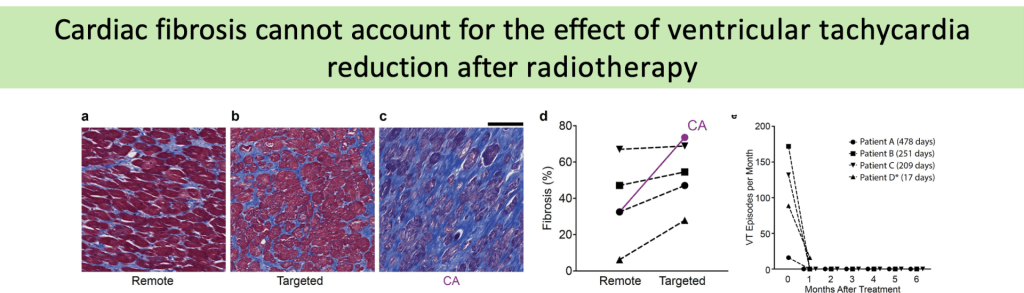
(a-c) Representative trichrome stains of remote (a), targeted (b), and failed catheter ablation (c)myocardium in a patient who received 25 Gyradiation for treatment of VT. Scale bars = 500µm.
(d) Percent fibrosis at time of heart explant from 4 patients who received SBRT in remote (left) and targeted (right) regions of myocardium.
(e) Defibrillator-recorded episodes of VT in the month before treatment and the 6months after treatment in these patients.