Global Centers
PET Radiotracer Translation and
Resource Center
The PET Radiotracer Translation and Resource Center (PET-RTRC) is a U.S. innovation hub for the development of novel PET radiotracers. Leading the way for an international network of collaborators, the PET-RTRC seeks to expand the understanding of diseases and advance the mission of precision imaging.

Our People
The PET-RTRC is a national laboratory for the advancement of precision imaging that brings together known innovators in the field of imaging.
Projects
The PET-RTRC offers collaborative and technology research and development (TR&D) projects to advance the development of PET radiotracers. We also have service projects that provide access for our partner institutions to mature center products otherwise not available.

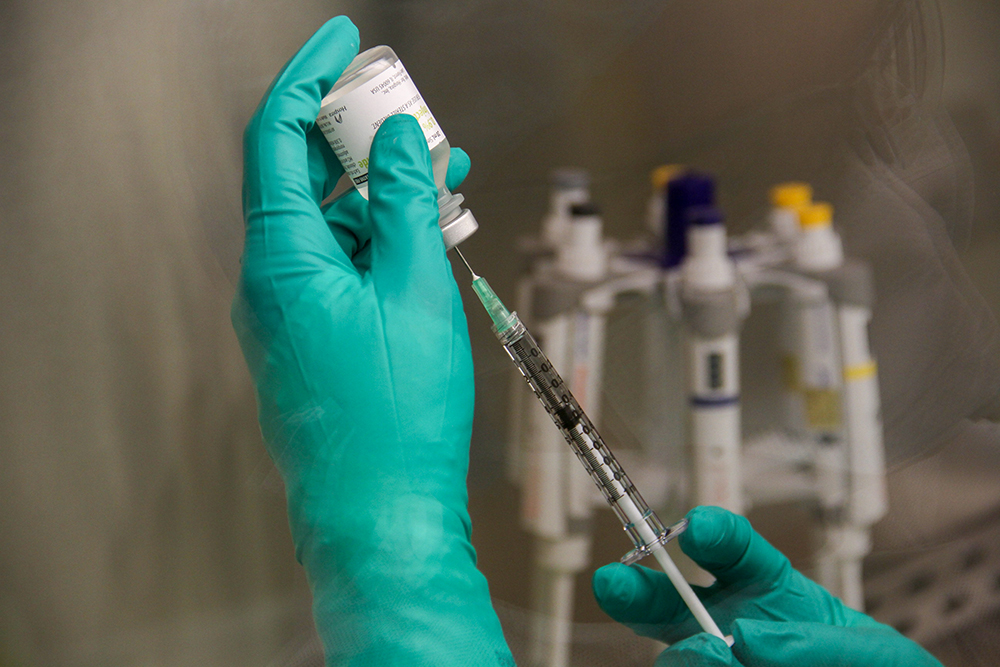
Resources
From radiotracers still in development to SOP’s and training for mature products, the PET-RTRC offers a wide variety of resources to support your projects.
Updates
Find out about upcoming news and events in the PET-RTRC, including lectures, workshops and training sessions.

Funding
The National Institute of Biomedical Imaging and Bioengineering (NIBIB) aims to improve health by leading the development and accelerating the application of biomedical technologies. This project is funded by NIBIB P41 EB025815.
NIBIB Centers
Other centers supported through the P41 grant include Resource for Molecular Imaging Agents in Precision Medicine at Johns Hopkins and Center for Molecular Imaging Technology and Translation at Harvard Medical School.

