Synapses consist of juxtaposed elements of two cells, the presynaptic and postsynaptic cell, which are specialized to send and receive chemical signals, respectively. In the case of neuromuscular synapses, these two specializations are separated by a specialized basal lamina of unique composition in the synaptic cleft.
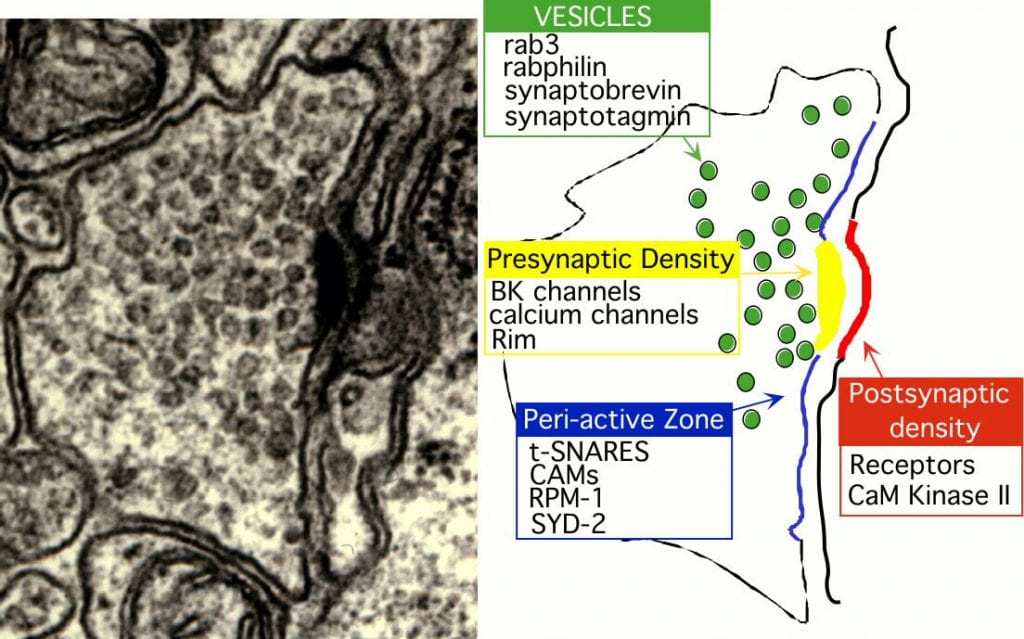
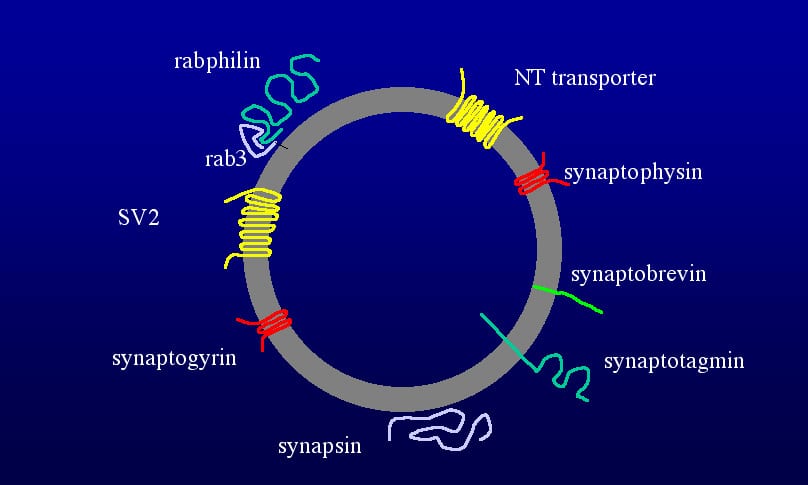
The most obvious constituents of the presynaptic specialization are the large cluster of synaptic vesicles and an active zone or presynaptic density that is associated with the site of vesicle fusion. Mitochondria are also often closely associated with presynaptic specializations as are endosomes. Each of these morphological elements has uniques protein components. Synaptic vesicles are highly inriched in a small set of periferally associated and integral membrane proteins. Most of the abundant proteins of synaptic vesicles have been moleculary characterized. They include synapsins, synaptophysin, synaptotagmin, rab3, rabphilin, synaptobrevin, synaptogyrin, the vacualor proton pump, neurotransmitter transporters and the proteoglycan SV2 (Figure 2). The role of some of these proteins in regulating synapse function is well characterized.
Another group of proteins are associated with domains at the plasma membrane of synapses. One such domain is the presynaptic density or active zone. Recent work has also defined a second domain surrounding the active zone called the peri-active zone. Several critical components of the release machinery are associated with the presynaptic density including calcium channels that directly flow a calcium into the nerve terminal upon depolarization, UNC-13 and RIM which direct priming of synaptic vesicles, as well as other proteins such as ELKS and liprin. Another set of proteins found at the synapse are associated with the peri-active zone, the area surrounding the presynaptic density. These proteins include RPM-1 and cell adhesion molecules.
A key question regarding synapse development and organization which remains largely unanswered is how all these proteins are targetted and maintained in these specialized domains. My lab is addressing this issue by studying the trafficking, targeting and synaptic localization mechanisms for several synaptic proteins.