Green fluorescent protein (GFP; Figure 1)and simlar proteins have been a wonderful addition to the tools we use to study synapses. These proteins are relatively small protein that fluoresce when irradiated by light of specific wavelenth.
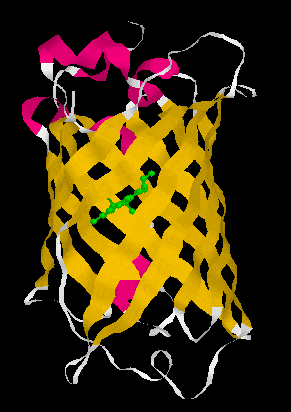
Figure 1. A GFP crystal structure showing it forms a ß-can structure (left) consisting of 11 anti-parallel ß-strands which form a cylinder. Loops (white) and a little bit of alpha helical structure (pink) connect the 11 ß-strands. The chromophore, p-hydroxybenzylidene-imidazolidone (in green), is formed via a post-translational oxygen-requiring reaction with contributions from the Ser65-Tyr66-Gly67 tripeptide. GFP is a very stable molecule and the chromophore is well protected and in a very insulated local environment in the middle of the cylinder. Surprisingly, deletion of more than the N-terminal Met or more than seven amino acids from the C-terminus leads to a complete loss of fluorescence. Nevertheless, both N- and C- terminal fusions are fluorescent consistent with the N- and C-terminus of GFP being flexible enough not to disrupt the general structure of the molecule. Structure:1EMB from PDB displayed with RasMol 2.6
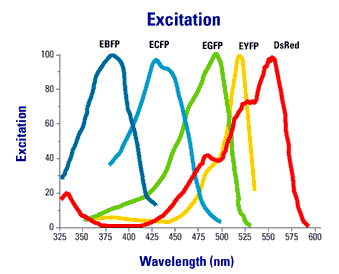
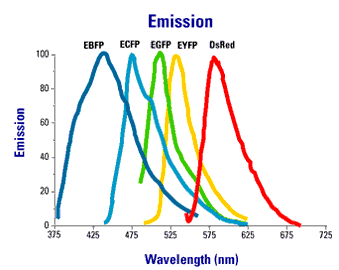
Using spectral variants of GFP (Figure 2), one can create double labeled strains that tag different components of the synapse simultaneously.
Figure 2. Excitation an emission spectra of commonly used fluorescent proteins.
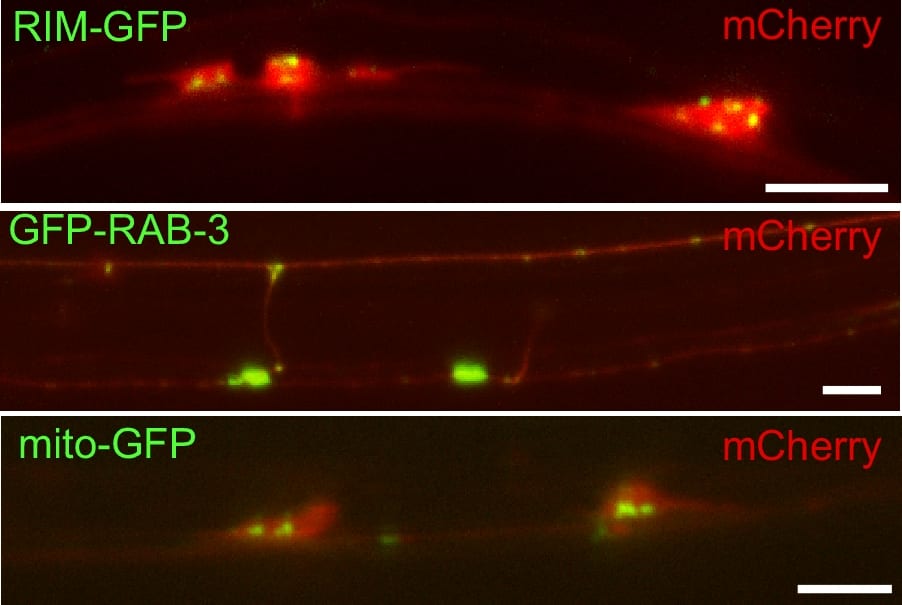
We have created several different GFP fusions to synaptic proteins that permit us to examine synapses. These include synaptobrevin-GFP, synaptogyrin-GFP and GFP-RAB-3. The most widely used of these is synaptobrevin-GFP which is used in cell culture, worms, and flies. The brightest of these fusions in our hands is GFP-RAB-3. In this case, GFP is added the N- instead of C-terminus because RAB-3 is post-translationally modified with the addition of a geranylgeranyl hydrophobic moiety to C-terminal cysteines which act as its anchor to the synaptic vesicle membrane. Because GFP:-RAB-3 is so much brighter we can now use the dissecting microscope to see certain sets of synapses in C. elegans . By expressing the GFP tag in different subsets of neurons using appropriate cell specific promoters we can examine different subsets of synapses. We mostly work with mechanosensory neurons (Figure 3).
Figure 3. Labeling synaptic compenents with fluorescent proteins in touch receptor neurons. Shown are three examples of double labeling between unc-10(Rim)-GFP to label active zones, GFP-RAB-3 to label synaptic vesicles, and mito-GFP to label mitochondria. All are double labeled with a cytosolic expressed mCherry fluorescent protein. Scale bar 5 um.
We have also developed tags for other compartments of the presynapse such as the active zone and mitochondria. Other groups working working with C. elegans and on other organisms have developed GFP-tagged neurotransmiter receptors and scaffolding proteins as markers for the postsynapse. These postsynaptic tags include the glutamate receptor subunit GLR-1, the acetylcholine receptor subunit UNC-29, the GABA receptor UNC-49 and scaffolding proteins like LIN-10.