Exploring Photosynthetic Protein Complexes:
Structure, Dynamics, & Regulation
Overview:
Our research seeks to broadly understand the structure and dynamics of biological macromolecules that dictate the solar energy capture and transformation. This area of research spans a range of topics and is well represented in the modern biophysics themes that interface with informational quantification/digitization era. Current areas of research include how membrane bound protein complexes that are involved in energy capture and transduction interact to carry out their chemical reactions, and how the membrane protein complexes change conformation to generate structural queue in synergy with other protein complexes in a larger scale energy flow pathway. By using state-of-the-art mass spectrometers coupled with in-depth biochemical/gene engineering technologies, we take a systematic approach to study photosynthetic reaction centers (RCs) dynamics upon changing environmental schemes, such as light and temperature. We strive to translate our structural findings into light/environmental adaptation context.
1. Dynamics of Light Driven Electron Transfer Networks to Environmental Changes.
Photosynthesis is the central biological process that is the source of energy for all photosynthetic organisms and, via the food chain, for almost all other forms of life. A fundamental challenge for plant biochemists is to understand how thylakoid membrane protein complexes respond to their environment to maintain/optimize their function. We target at intrinsic membrane domain level and their structural capabilities to accommodate
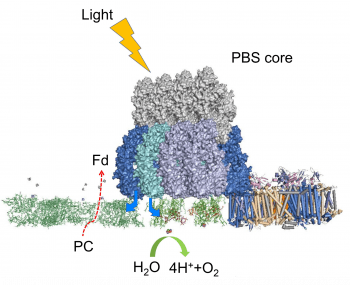
changes to form alternative (sub)optimum, and what’s their structural inflection point upon extreme environmental/ drastic developmental changes. A major goal of this program is to identify new protein complex organization of Photosystem II (PSII), Photosystem I (PSI), Cytochrome b6f complex, and ATP synthase, etc. Although, much is known about the classical function/regulation of these protein complexes at individual levels, multiple studies suggest that higher order protein structure of these complexes play important roles in orchestrating fine adjustment to meet cellular energy demands. We aim to develop protein residue level view of how these key protein complexes function in the context of varying conditions by using protein foot-printing in combination with mass spectrometry.
2. Molecular Mechanism of Action of the Cyanobacterial Orange Carotenoid Protein (OCP)
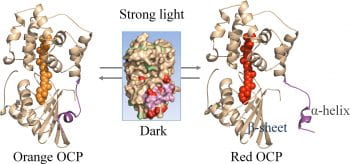
All photosynthetic organisms contain an antenna system that collects energy and transfers it to reaction centers (RCs) where chemical reactions store a portion of the energy in stable products. Photosynthetic organisms also invariably employ a number of protection and repair mechanisms to prevent and recover from excess light absorption. These mechanisms are as varied as the different types of antenna systems found in several classes of photosynthetic organisms. Photosynthesis protection mechanism, of key importance for understanding energy collection and storage in photosynthetic systems, is the theme of this program. Under saturating-light conditions, excess absorbed energy is dissipated as heat in a process called Non-Photochemical Quenching (NPQ). Two proteins, the Orange Carotenoid Protein (OCP) and the Fluorescence Recovery Protein (FRP), are involved in the process of PBS fluorescence quenching and recovery in cyanobacteria, however, the mechanistic details of the process have not yet been elucidated. how do the physiological conditions/factors facilitate the photoactivation of OCP? How does the binding pocket relays pigment changes to the global surface helical or loop structure to ready the OCP for PBS binding and quenching? and in what time scale? Is binding of OCPR to PBS sufficient for quenching? How is 3′-hECN oriented in a quenching complex of OCP-PBS? Where is OCPO sequestered in the cell under low light conditions? Note that no single technique is powerful enough to address various structural questions in a comprehensive and complete way, but we argue that significant advances can be made with MS-based approaches that are often termed “structural proteomics”.
3. Structural Synergy of Photosynthetic and Respiratory Electron Transfer in Cyanobacteria
The thylakoid membranes of cyanobacteria are the major sites of respiratory electron transport as well as photosynthetic light reactions. The uniqueness comes from that the fact that photosynthetic and respiratory electron transport chain share some components, how these two events and some common components coordinate to meet the cellular energy requirements remains poorly understood. Respiratory electron transport takes electrons derived from the catabolism of stored metabolites and pass them to an external electron acceptor. Cytochrome b6f is one of the major components shared between respiratory electron transport chain and photosynthetic transport chain, since reduced plastoquinone from PSII and Quinone oxidoreductase will be oxidized through Cytochrome b6f complex, which shares similar structure and function to Cytochrome bc1 complex of mitochondria. My proposed research in this plan will tackle the crosstalk between two energy flow pathways mediated by Cytochrome b6f complex and NDH complex. This research activities involve extremely diverse concepts and various disciples as I have been used and will be used, such as atomic force microscopy to probe the structure and dynamics of the protein complex apparatus. The knowledge generated by this work can be used for tailoring the physiology process related to the effective utilization of excitation energy by photosynthesis and can increase the physiological potential of thylakoid membrane potential of the green world
