Ion Channels: structure, function, and physiological roles
The fundamental importance of ion channels
Ion channels are the key proteins of cell membranes responsible for all the diversity in electrical signaling found in nerve and muscle cells, and virtually every living cell. A large number of genes are devoted to encoding ion channels and each kind of channel has very specific functional properties designed for specific particular physiological roles in the cells in which they are found. The suite of ion channels found in a particular cell, therefore, provides the electrical signature that defines the behavior of that cell. The set of ion channels in heart muscle define the long duration and repetitive nature of cardiac action potentials. The set of ion channels in particular nerve cells determines whether the cell fires repetitively at high frequencies or only transiently.
The voltage-gated potassium (K+) channel family include the largest number of distinct kinds of ion channels. In fact, of all genes that encode ion channels, the number of genes that encode K+ channels is the largest. Furthermore, there are additional mechanisms by which diversity in K+ channel function can be generated. Thus, the large variety of K+ channel types that are available for expression in cells attests to the central importance that K+ channels play in defining the electrical properties of cells.
K+ channels play critical roles in defining action potential frequency and duration, and repetitive firing properties of cells. Dysfunctions of particular K+ channels have been implicated in epilepsy, ataxias, and various cardiac arrhythmias. Regulation of K+ channel function and expression contribute to such fundamentally important processes as learning and memory. Thus, understanding the physiological roles of various K+ channels and the structural and functional properties of K+ channels will unquestionably continue to provide new insight into disorders of cellular electrical activity and insight into the fundamentally unique properties of nervous system
BK channels are a widely expressed K+ channel family of central importance in defining many key cellular electrical behaviors.
The focus of this laboratory has been on understanding the physiological roles and functional properties of one particular K+ channel family that is regulated not only by changes in membrane potential but also by changes in the concentration of calcium (Ca2+) inside of cells. These channels have a uniquely large single channel current so are often termed BK channels for Big K+ channels. BK channels are expressed among a wide variety of cells including almost all nerve cells, muscle cells, and endocrine cells. Among different cells, they play key roles in regulating action potential duration, action potential firing, secretion of hormone, and muscle relaxation.The dual regulation of BK channels by two important physiological signals, voltage and Ca2+, means that these channels can contribute to regulation of electrical activity over a broader range of physiological conditions than their strictly voltage-dependent K+ channel brethren. Thus, dependent on elevations of Ca2+ in a particular cell, BK channels can play a wide variety of physiological roles, all concerned with regulation of electrical excitability. Thus, in vascular smooth muscle, BK channels play a central role in the regulation of blood pressure by regulating muscle tone, while in neurons BK channels participate in fast action potential repolarization.
The work in this laboratory addresses two distinct general issues concerned with the properties of BK channels.
First, we are interested in the physiological roles that the BK channel family play among different cells. This work has involved identification of novel auxiliary subunits that regulate BK channel function (Xia et al., 1999; Xia et al., 2000) and involved studies of the contributions that BK channels make to cellular electrical properties (Solaro et al., 1995; Prakriya and Lingle, 1999; Prakriya and Lingle, 2000).
Second, we use BK channels to address fundamental issues concerned with the structural mechanics of how ion channels work. This work involves studies of the mechanism of inactivation of BK channels (Solaro et al., 1997; Ding et al., 1998; Lingle et al., 2001) and also how BK channels are regulated by both voltage and Ca2+ (Zeng et al., 2001; Zhang et al., 2001; Xia et al., 2002)
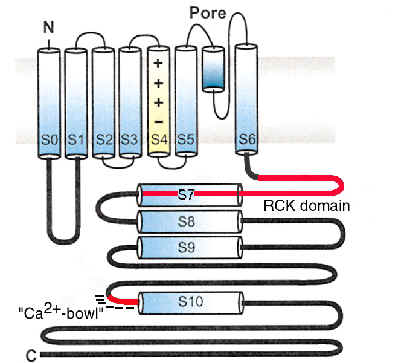
Schematic Diagram of the a subunit of BK channels
How is the functional diversity of BK channels generated? Auxiliary b subunits define many important properties of BK channels
BK channels are remarkable in that, unlike other voltage-activated K+ channels, they are all encoded by a single gene. Yet, among different cells BK channels exhibit a broad range of functional properties. One important question is therefore, how is the functional diversity of BK channels generated.
One early answer to this question was that, despite the single gene that encodes BK channels, the gene structure contains multiple coding fragments that can be assembled in distinct ways to form alternative mature subunits. However, the functional diversity that resulted from various alternatively spliced forms of BK channel subunits was not sufficient to account for the diversity found in cells.
Now it is recognized that an auxiliary subunit family, the b subunits, are a major determinant of the functional properties of BK channels among different cells. For example, in smooth muscle the b1 auxiliary subunit results in BK channels which are more sensitive to Ca2+ than BK channels found in neurons. This is a key factor in allowing BK channels to play in important role in smooth muscle relaxation.
The auxiliary b subunit family now includes four distinct genes (b1-b4) of which this lab has contributed to cloning, identification and characterization of two (b2 and b3, Xia et al., 1999; Xia et al., 2000).
In fact, our work has demonstrated the beta subunits define such diverse aspects of BK channel function as inactivation (Xia et al., 1999; Xia et al., 2000), single channel conductance (Wang et al., 2002), and apparent Ca2+-dependence (Xia et al., 1999; Xia et al., 2000; Wang et al., 2002), and current rectification (Zeng et al., 2001). This effects are particularly remarkable since such properties are usually thought to arise from the main pore-forming a subunits of a channel, and not from auxiliary b subunits.
A key question that remains to be resolved is the functional roles played by b subunits in defining the properties of BK channels in native cells.
Regulation of BK channels is controlled by two physiological signals: membrane voltage and cellular Ca2+
For most voltage-dependent ion channels, channel activation is controlled by a single physiological signal: membrane voltage. In contrast, two physiological stimuli regulate the activity of BK channels: membrane voltage and cytosolic [Ca2+]. In the absence of Ca2+, BK channels can be almost fully activated with extreme (non-physiological) depolarizations in a manner similar to activation of voltage-dependent K+ channels. However, elevations in cytoplasmic [Ca2+] are needed to shift the range voltages over which currents are activated to more negative potentials within the physiological range. This ability of both cytosolic [Ca2+] and voltage to regulate channel function allows BK channels to serve distinct physiological functions ranging from rapid repolarization following action potentials to control of action potential firing frequencies, contingent upon the [Ca2+] to which the channels are exposed.
A remarkable characteristic of the regulation of BK channels by Ca2+ is that current activation can be shifted by changes in [Ca2+] over a range from at least 0.5 mM through 50 mM . This broad range of Ca2+ sensitivity is unusual among Ca2+-regulated proteins, and the mechanisms that account for this regulation by Ca2+ remain poorly understood.