DNA methylation in the gene body influences MeCP2-mediated gene repression
Kinde BZ, Wu DY, Greenberg ME, Gabel HW. PNAS. 2016 Dec 27;113(52):15114-15119. doi: 10.1073/pnas.1618737114. Epub 2016 Dec 13.
Abstract
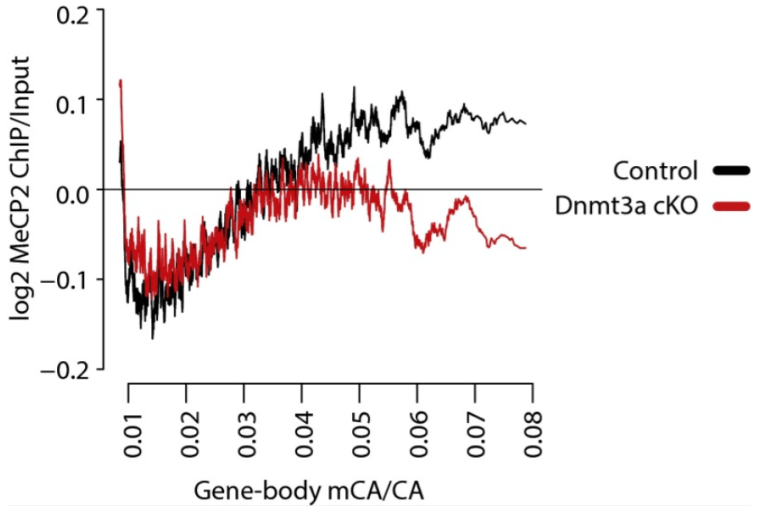
Rett syndrome is a severe neurodevelopmental disorder caused by mutations in the methyl-CpG binding protein gene (MECP2). MeCP2 is a methyl-cytosine binding protein that is proposed to function as a transcriptional repressor. However, multiple gene expression studies comparing wild-type and MeCP2-deficient neurons have failed to identify gene expression changes consistent with loss of a classical transcriptional repressor. Recent work suggests that one function of MeCP2 in neurons is to temper the expression of the longest genes in the genome by binding to methylated CA dinucleotides (mCA) within transcribed regions of these genes. Here we explore the mechanism of mCA and MeCP2 in fine tuning the expression of long genes. We find that mCA is not only highly enriched within the body of genes normally repressed by MeCP2, but also enriched within extended megabase-scale regions surrounding MeCP2-repressed genes. Whereas enrichment of mCA exists in a broad region around these genes, mCA together with mCG within gene bodies appears to be the primary driver of gene repression by MeCP2. Disruption of methylation at CA sites within the brain results in depletion of MeCP2 across genes that normally contain a high density of gene-body mCA. We further find that the degree of gene repression by MeCP2 is proportional to the total number of methylated cytosine MeCP2 binding sites across the body of a gene. These findings suggest a model in which MeCP2 tunes gene expression in neurons by binding within the transcribed regions of genes to impede the elongation of RNA polymerase.