Overview: This tutorial discusses limiting reagents, demonstrates how theoretical and percent yields are determined, and presents possible factors that may preclude the achievement of the full theoretical yield in practice.
Skills:
- Identifying the limiting reagent
- Calculation of theoretical and percent yields
New terms:
- Limiting Reagent
- Theoretical Yield
- Percent Yield
Typically in chemical reactions between two reagents, both are not used completely. In general, one may be used completely while some amount of the other reagent(s) may remain after the reaction has occurred. Those that remain are said to react in excess. The reactant that is used completely is the limiting reagent. The limiting reagent determines the amount of product(s) at the end of the reaction as well as how much remains of the reactant(s) that occurred in excess. When the limiting reagent is completely used the reaction stops. Why would chemists be interested in this information? Answer
In order to determine which reactant is the limiting reagent, take each reactant separately and assume that it is the limiting reagent. The reactant that produces the least amount of product must be the limiting reagent.
Example.
Tin (Sn) and iodine (I2) react to produce tin iodide (SnI4). If there are 10.00 g of tin and 40.00 g of I2 at the beginning of the reaction, what is the maximum amount of SnI4 that may be produced?
What is happening in this reaction? We have iodine, which is a black crystalline solid reacting with tin (a grey-white solid metal solid) to form SnI4. So the skeleton equation is Sn + I2–>SnI4.
Determine the Limiting Reagent: The first step is to balance the reaction: Sn + 2 I2–>SnI4. In this case, we do not know which reactant is the limiting reagent. In order to find how much product is formed, however, we must determine this. There are many ways to determine the limiting reagent. We will show one method that involves assuming one reactant is the limiting reagent and determine whether or not there is a sufficient amount of the other reagent(s) for the assumed limiting reagent to react completely. So to start let’s look at Sn and assume for now that it is the limiting reagent. How much iodine is needed to completely consume 10.00 g of Sn?
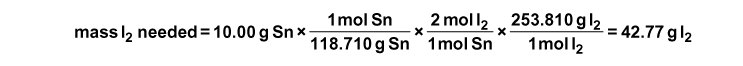
This means that in order for 10.00 g of Sn to be used completely, 42.77 g of iodine is needed. But we only have 40.00 g of I2 (i.e., not enough I2 to completely react with all of the tin). This means iodine is the limiting reagent.
Determine the amount of SnI4 that may be produced: We must use iodine for this calculation since it is the limiting reagent.

Prove to yourself that iodine is the limiting reagent. How much product could be produced from 10.00 g of Sn (assume that tin is the limiting reagent)? Answer
Determine the amount of compounds remaining when the reaction is complete: What remains in the reaction container after the reaction has been completed? There is no iodine, only Sn and SnI4. We have already calculated how much SnI4 is produced, but how much tin is left?

So after the reaction has taken place, we would expect 49.35 g of SnI4 and 0.65 g of Sn in the reaction container. An alternative approach to answer this question.
Determine the Yield: However, when the reaction actually occurred, only 25.8 g of SnI4 was obtained. What happened? Why isn’t there 49.35 g of SnI4? What was obtained from the experiment was the actual yield and the mass we calculated (49.35 g SnI4) is the theoretical yield. From this we can calculate the percent yield.

What is the percent yield in our experiment?
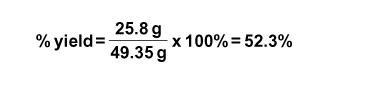
Reasons for not achieving the theoretical yield
What are some possible reasons why the theoretical yield is not achieved? Answer
Advanced Applications:An organic chemist at Washington University develops methods for synthesizing pharmacologically active compounds.
Summary
After completing this review, you should be able to determine the limiting reagent and be able to calculate the amount of product formed from the limiting reagent. You should also understand the percent yield of the reaction.
Practice Problems
- 1. Take the reaction: NH3 + O2 –>NO + H2O. In an experiment, 3.25 g of NH3 are allowed to react with 3.50 g of O2. Hint
- a. Which reactant is the limiting reagent?
- b. How many grams of NO are formed?c. How much of the excess reactant remains after the reaction?
- 2. If 4.95 g of ethylene (C2H4) are combusted with 3.25 g of oxygen. Hint
- a. What is the limiting reagent?
- b. How many grams of CO2 are formed?
- 3. Consider the reaction of C6H6 + Br2 –>C6H5Br + HBr
- a. What is the theoretical yield of C6H5Br if 42.1 g of C6H6 react with 73.0 g of Br2?
- b. If the actual yield of C6H5Br is 63.6 g, what is the percent yield?
- 4. Use the following reaction: C4H9OH + NaBr + H2SO4 –> C4H9Br + NaHSO4 + H2O If 15.0 g of C4H9OH react with 22.4 g of NaBr and 32.7 g of H2SO4 to yield 17.1 g of C4H9Br, what is the percent yield of this reaction? Hint
- 5. Silicon nitride (Si3N4) is made by a combining Si and nitrogen gas (N2) at a high temperature. How much (in g) Si is needed to react with an excess of nitrogen gas to prepare 125 g of silicon nitride if the percent yield of the reaction is 95.0%? Hint
- 6. Souring of wine occurs when ethanol is converted to acetic acid by oxygen by the following reaction: C2H5OH + O2 –> CH3COOH + H2O. A 1.00 L bottle of wine, labeled as 8.5% (by volume) ethanol, is found to have a defective seal. Analysis of 1.00 mL showed that there were 0.0274 grams of acetic acid in that 1.00 mL. The density of ethanol is 0.816 g/mL and the density of water is 1.00 g/mL.
- a. What mass of oxygen must have leaked into the bottle? Hint
- b. What is the percent yield for the conversion of ethanol to acetic acid if O2 is in excess? Hint
- 7. A reaction container holds 5.77 g of P4 and 5.77 g of O2.The following reaction occurs: P4 + O2 –> P4O6. If enough oxygen is available then the P4O6 reacts further: P4O6 + O2 –> P4O10. Hint
- a. What is the limiting reagent for the formation of P4O10?
- b. What mass of P4O10 is produced?
- c. What mass of excess reactant is left in the reaction container?