Overview: This section introduces the properties of waves and wave motion. A number of fundamental physical phenomena are describeable as waves. In Chemistry 111A you will be introduced to quantum mechanics, which is also sometimes called wave mechanics.
Skills:
- Relating wavelength, frequency and wave velocity
- Simple problems involving constructive and destructive interference
New terms:
- Amplitude
- Wavelength
- Frequency
- Harmonic
- Superposition
In the last tutorial section, we introduced electric fields and the notion that opposite charges attract. In the very first tutorial, The Atom, we saw that electrons in an atom remain outside the nucleus. Even though there is a positive charge inside the nucleus, the electrons do not collapse into the nucleus. Why don’t the electrons simply fall into the nucleus and neutralize the positively charged protons? This question baffled many great scientists for a number of years. Classical physics cannot describe the details of the atom very well. It was not until quantum mechanics emerged in the early 20th century that a more detailed description of the atom’s behavior was possible. According to classical physics, matter exhibits particle-like behavior and wave phenomena are treated as a separate discipline. Quantum mechanics shook things up by saying that matter and energy, including light, has both particle and wave properties, a dual wave-particle character. This dual character is more pronounced in small particles, such as electrons in the atom. The proof that quantum mechanics is correct will come in Chemistry 111. Right now we will simply discuss waves and their properties.
We use amplitude, frequency and wavelength to describe waves. What is each of these and for that matter, what do waves look like?
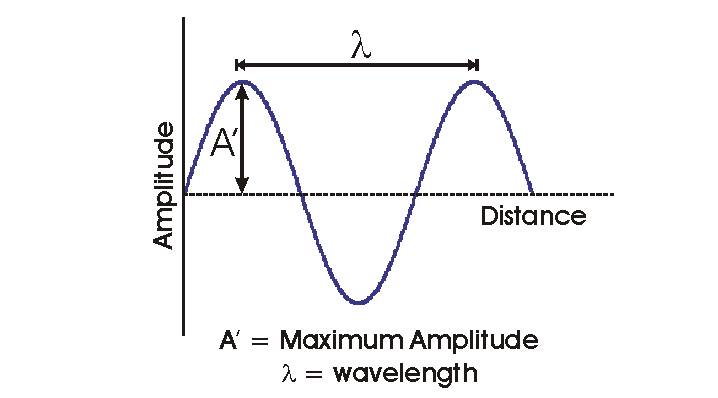
The amplitude is the maximum displacement of the wave. The wavelength is the distance between two successive identical points in the wave cycle (for instance, two successive peaks or troughs in the wave).
Waves may propogate in both space and time. Frequency is a measure of how the wave propogates in time. Frequency is the number of wave crests (or successive identical points in the wave cycle) to pass a fixed point in one second.
There are two types of waves that we will discuss. These are the harmonic (standing wave) and the non-harmonic (traveling wave). A traveling wave is one that changes its shape over time. Traveling waves start at one point and are propogated outward freely. They are not restricted beyond the one fixed starting point. Think about the waves that are produced when a rock is thrown into a pond. They start at the point where the rock entered the water and propogate outwards. Eventually the waves broaden out and have a smaller maximum amplitude. This is due to the fact that there are multiple frequencies in a traveling wave (you don’t need to know why right now, you just need to know what a traveling wave is). A standing wave is different. It is fixed at two places. When a wave is created, it starts from a fixed starting point and travels to the other fixed point. Once it reaches the second fixed point, the wave gets carried back (is reflected back) to the starting point. Imagine shaking a rope that is tied down at one end. The wave, after traveling down the rope, returns back to the starting point. In this case, the wave does not change over time. Every wave will eventually have the same frequency.

Imagine the darker line traveling to the left. It starts at zero, and travels to the other side. Once it reaches the other fixed point, the wave travels back to the starting poing, this is represented as the lighter line. Notice the point right in the middle at p. This point is called a node. When the wave reaches a crest, it is referred to as an antinode. In this particular graph, the wave is the second harmonic. The number of nodes plus one is the harmonic of that wave. [The points of fixed zero amplitude at the ends of the standing wave are not counted as nodes.] At the node, there is no movement. So if we take this to be a rope, that rope does not move at the node.
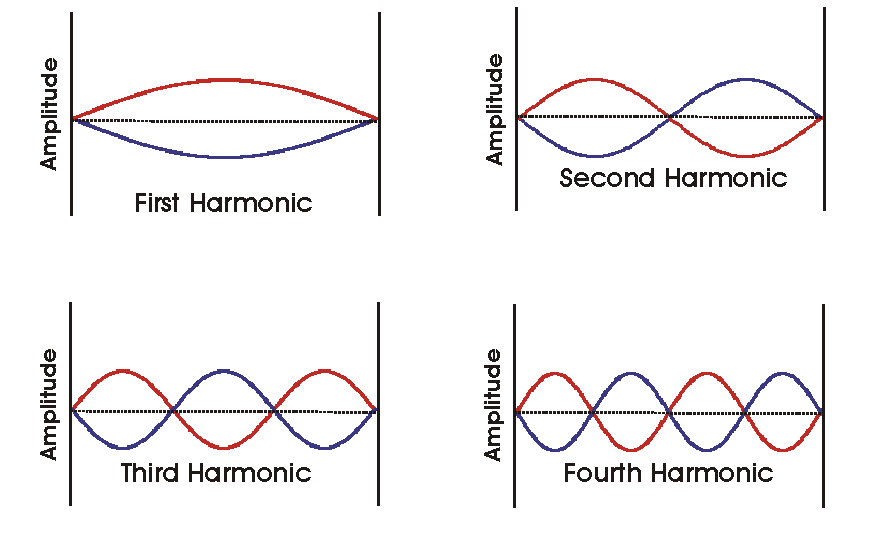
How many nodes does the first harmonic have? Answer
How many nodes do the third and fourth harmonics have? Answer
How many nodes does the seventh harmonic have? Answer
Each standing wave has a particular wavelength based on the length of the fixed region. The equation is:
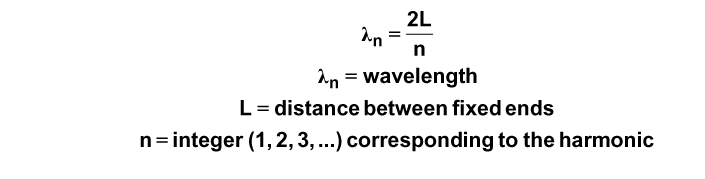
For a wave, the speed of propogation is related to wavelength and frequency of the wave.

Light Waves
Light waves are different from classical waves such as sound and water waves. Classical waves are described as a disturbance that transfers energy from point to point in a medium. However, light waves do not require a medium for their propogation. If this was not true and a medium was required for light wave propogation, we would not be able to see the stars. Space is a vacuum; there is no conducting medium in space through which light travels. Light waves still transfer energy and momentum, though, just as classical waves do. We see this in the form of solar energy and for plants in photosynthesis. Scientists refer to light as a form of electromagnetic radiation. Some examples of “light” or electromagnetic radiation are UV and visible light from the sun, microwave radiation used to cook food and infrared radiation from heat.
Electromagnetic Wave: A disturbance that transfers energy and momentum through space from one region to another, even in the absence of any medium in the intervening region.
These waves are referred to as electromagnetic waves because they consist of oscillating electric and magnetic waves which you will learn more about in general chemistry.
Light waves are characterized by amplitude, wavelength, and frequency just as classical waves are. The speed of light, c, is defined as 2.997925 x 108 m/s in a vacuum (this is very close to the value in air as well).

Superposition Principle
Waves can interfere with one another and be either constructive or destructive. In other words, waves either add to (reinforce) or subtract from (cancel out) one another. This is very important in chemistry because this is how chemical bonding works. A chemical bond is formed when electron waves constructively interfere. In general chemistry, you will learn more about this property of bonding.
When two waves are both positive in the same area, they will add constructively. The resulting wave will be the sum of the two individual amplitudes as shown below. This destructive interference is referred to as chemical antibonding.


This superposition principle allowed scientists to establish the wave character of light. You will learn about this experiment in Chemistry 111.
Summary
You should now have a better understanding of waves and their properties including amplitude, frequency, and wavelength. Also you should know about electromagnetic waves and how they differ from classical waves. You should be familiar with the superposition principle as well as harmonic waves.
Questions
You may not be able to answer the following questions yet, but keep them in mind as you go through general chemistry.
- In the tutorial module dealing with The Atom we noted that the electrons in an atom are distributed around the nucleus. One may wonder, as did classical physicists prior to the early 20th century, “Why don’t the electrons in the atom simply spiral in to the nucleus?”
- What do waves and the superposition of waves have to do with bonding? And what is “antibonding”?
- Why are electromagnetic waves so special?
- How do we know that quantum mechanics is right? In other words, how do we know that matter behaves both as particles as well as waves?