Overview: This section provides a review of chemical formulas and the information that is available from the different, but equivalent representations (formulas) of a molecule/compound.
Skills:
- Understand chemical formulas and the different representations of the chemical formula.
- Understand percent elemental composition
- Be able to determine the empirical formula from the percent elemental compositions and vice versa.
New terms:
- Chemical Formula
- Structural Formula
- Molecular Formula
- Empirical Formula
- Percent elemental composition
First what is a chemical formula? They have been used numerous times already in this tutorial but so far, they have not been defined.
Chemical Formula: A representation of the chemical composition of a substance. This can be either a molecular or empirical formula.
Basically a chemical formula gives scientists a variety of information about a particular compound.
Let’s look at iso-octane. Iso-octane is the component of gasoline that burns the smoothest. Iso-octane can be written in numerous ways.
Structural Formula

- Like the other formulas mentioned below, the structural formula tells us which atoms are in a given molecule. But, this type of formula also gives us information about the way those atoms are connected to one another. There are multiple ways to show the structural formula and each representation gives certain information about the compound such as the connectivity of the atoms or the molecules shape. To see some of the types of structural formulas that are available, click here.
- Molecular Formula
The molecular formula specifies the number of atoms of each element in one molecule. For iso-octane this is C8H18. This means that there are 8 atoms of carbon and 18 atoms of hydrogen in one molecule of C8H18. This also means that in one mole of iso-octane, there are 8 moles of carbon and 18 moles of hydrogen. - Empirical Formula
The empirical formula is the simplest whole-number ratio of atoms in the compound. For iso-octane, the empirical formula is C4H9. Notice that the molecular formula is twice that of the empirical formula. All molecular formulas are an integer multiple of the empirical formula for a compound.
FYI: Many compounds in nature have the same empirical formula and even the same molecular formula even though they have vastly different properties. For instance, formaldehyde, acetic acid (vinegar) and lactic acid (the acid in sour milk), glucose and galactose all have the same empirical formula of CH2O. The only difference between these molecules is the multiple of the empirical formula. However, I am quite certain that no one would mistake formaldehyde for glucose. Glucose and Galactose both have the same molecular formula, C6H12O6, but in the body, galactose must be first converted to glucose to make energy. The difference is their structures (i.e., how the atoms in each molecule are connected (bonded) to one another).
Percent Composition by Mass (Weight)
Experimentally, the mass percentage of a compound is obtained by means of combustion analysis or other types of elemental analysis. We can use mass percentages to determine empirical formulas, but not molecular formulas.
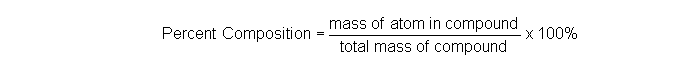
Please note that the weight fraction is equal to the mass fraction so that the percent weight also equals the percent mass. This is because weight and mass are proportional to each other.
Example 1.
What is the percent elemental composition by weight (mass) of each iso-octane molecule, C8H18?
What do we need to do first? We need to compute the mass of each of the elements, C and H, which can be done with the equation above and the information from the molecular formula. We know that in each molecule of iso-octane, there are 8 atoms of carbon and 18 atoms of hydrogen. This tells us that in each mole of iso-octane, there are 8 moles of C and 18 moles of H. By now, we can easily go from moles to mass by using molar masses. Unfortunately, we do not know how many moles of iso-octane there are. So what can we do? Remember what it is that we are looking for in the problem. We want the percent mass. The percent mass remains the same for iso-octane whether there is 1 molecule or 5000 moles of iso-octane. So to make things easy, we’ll assume that there is one mole of iso-octane. First we will find the mass of each element, then find the percent composition by dividing the molar mass of each element by the total mass of the molecule?

This means that iso-octane is 84.12% carbon. The percent hydrogen must be 100% – 84.12% since the total elemental percentages must add up to 100. Therefore hydrogen accounts for 15.88% of the molecular mass. Notice that we could have found the % H first and then subtracted to solve for the percent carbon. Try solving for the percent hydrogen first and then the percent carbon to verify that you get the same answer.
Once you have the percent elemental compositions, you can derive the empirical formula.
Example 2:
What is the empirical formula of a compound containing 84.12% carbon and 15.88% hydrogen by mass?
To find the empirical formula, we need to find the number of moles of each element. We are able to go from mass to moles by the molecular mass but we do not have an initial mass. We can assume a mass of the sample because, once again, the percent mass is the same no matter what the mass of the sample is. To make things easy, we will assume 100 g of compound. Therefore, there are 84.12 g (this is 84.12% of 100 g) of carbon and 15.88 g (15.88% of 100 g) of hydrogen.

Now determine the smallest mole ratio. Do this by taking the number of moles of the element over the number of moles of the element that has the least number of moles in the compound. In this case, carbon has the least number of moles.

Now we need to find the smallest integer ratio. Carbon is already in integer form but what factor multiplied by 2.25 gives an integer. The factor will be 4. This means we need to multiply each of these mole ratios by 4 to get the empirical formula.

From the information given in this example, can we determine the molecular formula?
Answer
Example 3:
Given that the molecular mass for a compound with an empirical formula of C4H9 is approximately 114 g/mole, what is the molecular formula of the compound?
We know the molecular formula is a multiple of the empirical formula: (C4H9)x
The molecular mass will be the sum of the individual molecular masses

Therefore the molecular formula is (C4H9)2 = C8H18, which is iso-octane.
Combustion Train
When a hydrocarbon, a compound that contains only carbon and hydrogen, is burned in oxygen, it yields CO2 and H2O. This is done using a combustion train. In the combustion train, the sample is weighed and then heated in the presence of oxygen. The products, carbon dioxide and water, are allowed to pass into another chamber where the water is absorbed. A dessicant such as magnesium perchlorate absorbs the water. The carbon dioxide continues into the final chamber where it is absorbed by NaOH and CaCl2. Once the original hydrocarbon is completely burned, the absorbers are weighed. The difference in masses is due to the CO2 and H2O (the H and C come only from the hydrocarbon). From this information, one can determine the empirical formula.
Example 4:
A certain hydrocarbon, when burned completely in oxygen, yields 3.38 g of CO2 and 1.384 g of H2O and no other products. What is the empirical formula of the compound?
Advanced Applications:A trip back in time. Advanced Applications circa 1890.
Summary
Now you should have a better understanding of chemical formulas and the different ways chemists represent a compound. Also, you should be able to determine percent elemental compositions and know how to calculate empirical formulas from the percent elemental composition.
Practice Problems
- 1. H3PO4, Phosphoric acid, is used in detergents, fertilizers, toothpastes and flavoring in carbonated beverages. Calculate the percent composition by mass to two decimal places of H, P and O in this compound. Hint
- 2. What is the percent composition by mass of aspartame (C14H18N2O5), the artificial sweetener NutraSweet?
- 3. Ascorbic acid (vitamin C) is 40.92% C, 4.58% H and 54.50% O by mass. What is the empirical formula of ascorbic acid? Hint
- 4. What is the empirical formula of each of the following compounds? Hint
a. Talc by mass composition contains 19.2% Mg, 29.6% Si, 42.2% O and 9.0% H.
b. Saccharin has by mass composition 45.89% C, 2.75% H, 7.65% N, 26.20% O and 17.50% S.
c. Salicylic Acid, used in aspirin, contains 60.87% C, 4.38% H, and 34.75% O by mass composition.
d. L-Dopa, a drug used for the treatment of Parkinson’s disease, is 54.82% C, 5.62% H, 7.10% N, and 32.46% O by mass composition. - 5. Determine the empirical formula of the following compounds that underwent combustion analysis.
a. Toluene is composed of C and H and yields 5.86 mg of CO2 and 1.37 mg of H2O after combustion. Hint
b. 0.1005 g of menthol, which is composed of C, H, and O, yields 0.2829 g CO2 and 0.1159 g H2O after combustion. Hint - 6. What is the molecular formula of benzoyl peroxide (the empirical formula is C7H5O2) if the molecular mass is 242 g/mol? Hint
- 7. What are the empirical and molecular formulas of the following compounds? Hint
a. Ibuprofen by mass composition is 75.69% C, 8.80% H and 15.51% O and the molecular mass is approximately 206 g/mol.
b. Caffeine contains by mass composition 49.5% C, 5.15% H, 28.9% N and 16.5 % O and the molecular mass is about 195 g/mol.