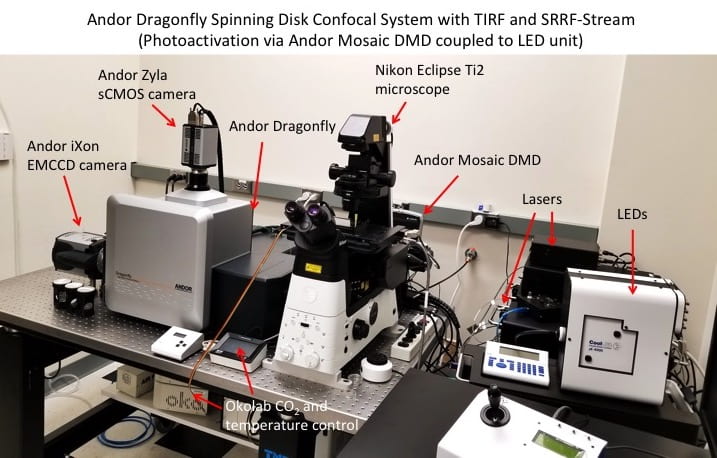
The system has the following components:
- Nikon Eclipse Ti2 microscope – automated inverted microscope controlled through Andor Fusion and iQ software. Equipped with high-NA objectives for confocal and TIRF imaging. Stage-top incubator allows for specified temperature and CO2 to be maintained over long periods.
- Andor Dragonfly 505 – spinning disk confocal unit with 25 µm and 40 µm pinhole sizes capable of high-speed and high-sensitivity imaging in confocal, widefield, and TIRF modes. Capable of super-resolution (SRRF-stream) imaging. Capable of rapid deconvolution of z-stacks and dual-camera image acquisition.
- Andor Mosaic DMD – a 800 x 600 array of microscopic tiltable mirrors which are digitally switched on/off to direct LED or laser light to a specific ROI in the imaging field. This allows for localized activation of optogenetic proteins, as well as photobleaching and photoswitching of fluorescent proteins. Capable of rapid switching speeds and simultaneous illumination of multiple ROIs.
- CoolLED pE-4000 LED unit – 16 selectable wavelengths ranging from 365 to 770 nm. Integrated with the Mosaic unit to illuminate specific ROIs with high spatiotemporal precision. Used for activation of optogenetic proteins and photoswitching/photoconversion of fluorescent proteins.
- Imaging lasers – five solid state lasers (445 nm, 488 nm, 514 nm, 561 nm, and 637 nm) contained in integrated laser engine (ILE) unit. Allow for imaging of a wide range of fluorescent proteins.
- Photobleaching laser – 445 nm 1.3 W laser integrated with the Mosaic unit for photobleaching of fluorescent proteins within a specific ROI.
- Andor iXon 888 Life EMCCD camera – high sensitivity (>95% QE) camera capable of single molecule detection. 1024×1024 pixel sensor. Cooled to -80°C to minimize thermal noise. The high sensitivity and fast frame rates make this camera ideal for SRRF-stream imaging.
- Andor Zyla sCMOS camera – 4.2 Megapixel (2048×2048) camera, 80% QE, frame rate of 53 frames/sec. Ideal for applications requiring high resolution imaging of bright signals.
- Okolab CO2 and temperature controllers – maintain the stage-top incubator at 37°C and 5% CO2 for live cell imaging experiments.
- Workstation – PC with dual quad-core Xeon CPUs, 96 GB ECC RAM, 6x SSDs (4TB for images), nVidia M4000 8GB and AMD W7100 8GB. Equipped with Andor Fusion software for rapid imaging and real-time visualization, iQ software for controlling Mosaic and LEDs, and Imaris software for image processing.
- Vibration isolation table

The system has the following components:
- Leica DMI6000 automated scope with motorized XYZ axis; DIC optics; epifluorescence ready – The scope is completely automated and controllable through attached computer. Inverted scope with 63x plan apochromat and 20x FluoR lenses. Multiple prism slots for visualizing multiple fluorophores through eye piece. The front panel switches allow unhindered control of various functions of the scope. The scope can also be independently operated without the computer. Full color images from the scope can also be captured using a Nikon D40 SLR attached to the right photoport of the scope. The left photoport is attached to the laser spinning disk confocal unit. The XYZ axes are controlled using a isolated controller.
- Environment incubation chamber – Specially designed for live cell imaging studies, the ‘On-scope incubator’ maintains the cells at 37oC, optimal CO2 concentration and humidity for long imaging experiments.
- Yokogawa spinning disk confocal unit – especially useful for live cell imaging studies on account of its ability to capture images at high speed without having undue photobleaching or phototoxicity effects. We possess multiple dichroics for imaging multiple fluorophores using this unit.
- Andor Revolution XD – A unit specifically designed for live cell confocal imaging with multiple laser lines. Integral device for combining multiple laser lines, their rapid switching and multiport delivery which allows same laser lines to be used for confocal imaging, FRAP and photoactivation experiments and TIRF.
- Solid state lasers – Multiple laser lines for imaging a variety of fluorophores. We possess 50 mW solid state laser lines of 454nm, 488nm, 515nm and 594nm wavelengths. Solid state laser allow rapid initialization of the lasers; do not generate heat and noise while operational and have a long life (up to 10,000 hrs). We have lasers from – Coherent and Cobolt Inc.
- Andor iXon EM–CCD camera – Integral for spinning disk confocal imaging on account of its higher sensitivity. Resolution 512 x 512k; upto 35 frames per second at maximum resolution; 99% quantum efficiency; cooled to -100oC to eliminate dark noise/ current; dual chip sensor for rapid frame transfer features. Capable of single – molecule imaging experiments.
- Revolution FRAPPA – A specially designed unit for FRAP and photoactivation (PA) studies in living cells. Capable of FRAP and PA with 10 msec time frames. Uses a dual galvanometer scan head to provide a computer-steered laser beam delivery system. Uses a unique ‘point ‘n shoot’ technique for FRAP and PA.
- Andor iQ – The software which runs all these units in perfect sync. User friendly and intuitive design. Uses a unique kinetic image disk for rapid acquisition and storing vast amounts of imaging data.
- Dell Optiplex workstation – Complementing the advanced imaging system, Intel Quad Core Duo processor; 4GB RAM; 1TB RAID 0 HDD; 2 TB secondary HDD; 30 inch digital monitor; wireless input devices; multiple external backup devices. Onboard DAC and laser controlled devices for generating rapid responses from attached devices.
- Vibration Isolation Table