Our primary goal is to unravel the molecular events that dictate the regenerative response of neurons in the peripheral nervous system and to relate this information to the lack of regenerative capacity in the central nervous system.
Cellular, Molecular and Epigenetic Control of Axon Regeneration
Permanent disabilities, including paralysis, numbness and pain following central nervous system (CNS) injuries result from the failure of injured axons to regenerate and re-build functional connections. The poor regenerative capacity of mature CNS neurons remains a major problem in neurobiology and an unmet medical need. There are currently no satisfactory treatments to restore mobility and sensation following spinal cord injury (SCI) or vision after optic nerve damage. In contrast, axon regeneration and partial functional recovery do occur in injured peripheral nerves, providing an opportunity to identify molecular mechanisms that control the axon regeneration program.
To understand why regeneration occurs in the peripheral but not the central nervous system, our lab studies a unique cell type that spans both systems: sensory neurons of the dorsal root ganglia. The cell bodies of sensory neurons are located in the dorsal root ganglion, a structure that sits just outside the spinal cord. These sensory neurons have a unique pseudo-unipolar morphology with a single axon which bifurcates within the ganglion; one axon proceeds along peripheral nerves and the other proceeds centrally along the dorsal root into the spinal cord. Importantly, the peripheral axon has a much greater regenerative capacity than the central axon.
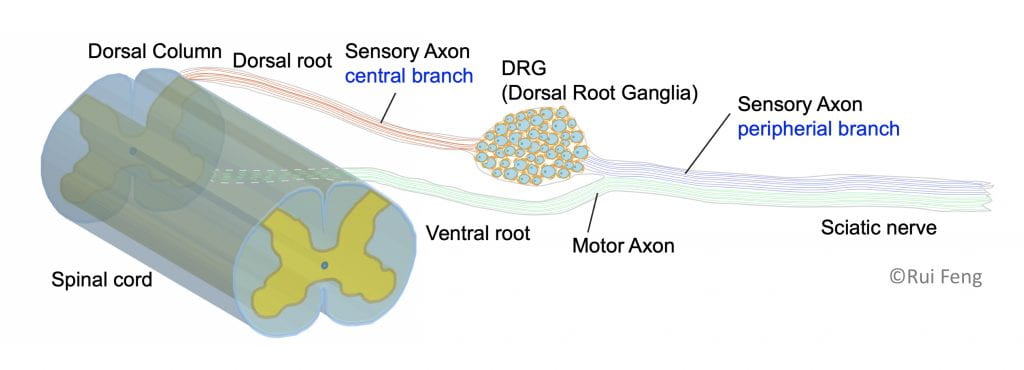
The primary goal in the Cavalli lab is to reveal the principles and mechanisms by which injured sensory neurons re-activate a pro-regenerative program following peripheral axon injury and identify potential targets for future treatment of CNS injuries and severe nerve injuries. Indeed, functional recovery in the peripheral nervous system is often incomplete, especially after complete nerve transection or when axons need to re-grow long distances to reach their targets.
We accomplish this goal using the mouse model system and apply a combination of biochemistry, cell biology, in vitro and in vivo imaging techniques and single cell sequencing approaches. Using human tissue, we also determine whether the findings made in the mouse model system are relevant to human physiology.
We hope to reveal strategies to initiate the pro-regenerative program and stimulate robust and meaningful long-distance axon regeneration in the injured nervous system.
Current Projects
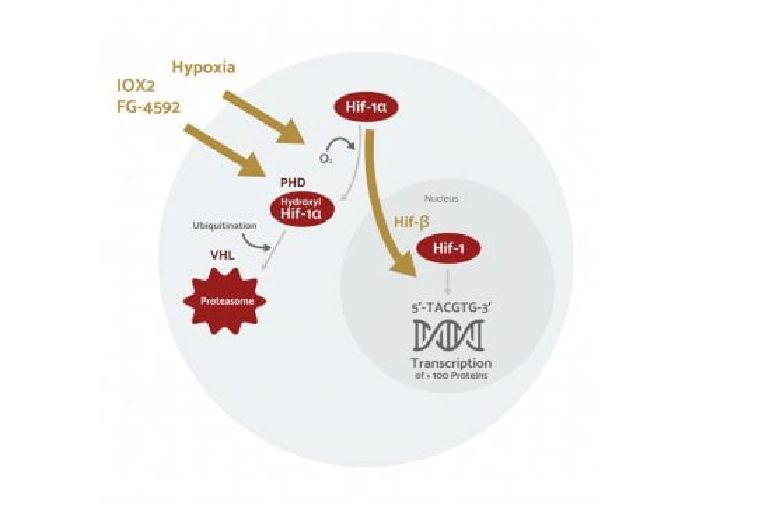
Intrinsic mechanisms controlling axon regeneration
We are studying the mechanisms by which a pro-regenerative state is reprogrammed following axon injury in sensory neurons.
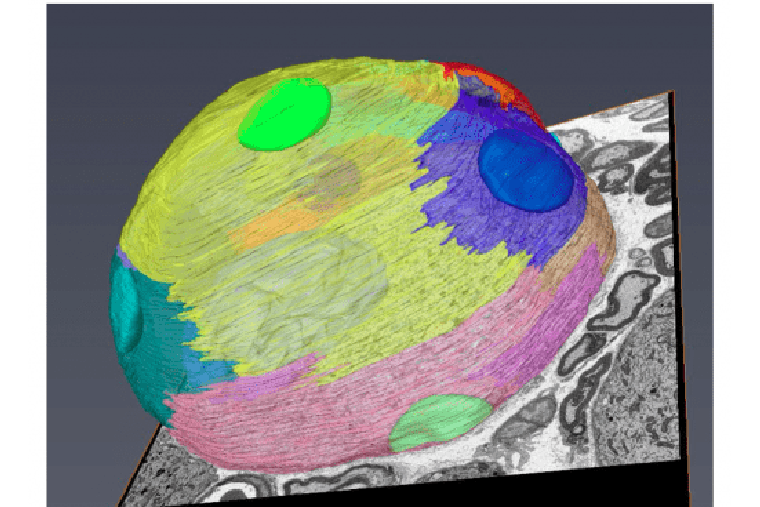
Role of the DRG microenvironment surrounding neuronal soma in axon regeneration
We are using single-cell RNAseq of DRG in naïve and injured conditions in vivo to unravel if and how non-neuronal cells respond to injury and actively contribute to the axon regeneration process.
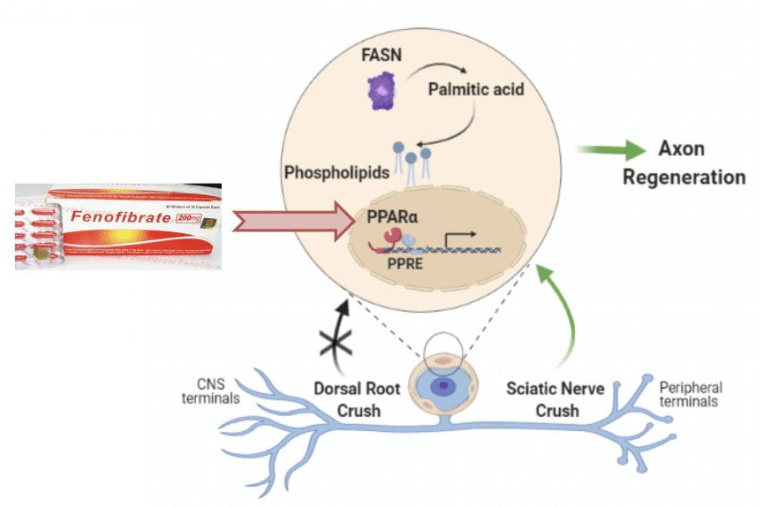
Targeted strategies to improve regeneration in the injured CNS
As we make progress in deciphering the pathways regulating axon regeneration in peripheral sensory neurons, we are testing whether manipulating neurons and non-neuronal cells, genetically or pharmacologically, enhances axon regeneration in the CNS, using both the spinal cord and the optic nerve injury models.
Collaborators at Washington University
- Ram Dixit, Dept. of Biology
- Stefanie Geisler, Dept. of Neurology
- Rob Gereau, Dept. of Anesthesiology
- Vitaly Klyachko, Dept. of Cell Biology and Physiology
- Mayssa Mokalled, Dept. of Developmental Biology
- Clay Semenkovich, Division of Endocrinology, Metabolism & Lipid Research
- Guoyan Zhao, Dept. of Genetics