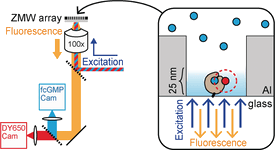
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels play critical roles for oscillatory neuronal activity and pacemaking in the heart. We are interested in understanding the mechanism by which cyclic nucleotide binding enhances HCN voltage-dependent activation. Toward this aim, we are studying ligand association events at the single-molecule level. However, a major challenge hampering resolution of single modest affinity binding events is the diffraction limit of light microscopy. At the high concentrations necessary to drive these binding reactions, the number of free fluorescent ligands within the diffraction-limited excitation volume becomes appreciable, thereby obscuring resolution of single binding events. To overcome this “concentration barrier”, we are using nano-fabricated waveguides to study ligand-binding dynamics and understand the mechanisms of ligand activation. These approaches allow us to resolve, for the first time, elementary ligand binding events in a ligand-activated ion channel.
- Goldschen-Ohm MP, Klenchin VA, White DS, Cowgill JB, Cui Q, Goldsmith RH, Chanda B. Structure and dynamics underlying elementary ligand binding events in human pacemaking channels. Elife. 2016 Nov 18;5PubMed PMID: 27858593; PubMed Central PMCID: PMC5115869.
- Goldschen-Ohm MP, White DS, Klenchin VA, Chanda B, Goldsmith RH. Observing single-molecule dynamics at millimolar concentrations. Angew. Chem. Int. Ed. doi:10.1002/anie.201612050