BRC
Functional Neuroimaging
and Biophotonics Lab
The Bauer Lab, also known as the Functional Neuroimaging and Biophotonics Lab, develops novel optical imaging technology for both basic and translational neuroscience applications in mice. Two major themes in our research are mapping functional brain organization in the mouse and examining how changes in local neural activity are related to corresponding changes in blood flow. We are particularly interested in understanding functional network connectivity in healthy mice, how it evolves following stroke, and endogenous mechanisms involved in stroke recovery. An important component of our research involves collaboration with clinicians to discuss how our findings at the bench relate to observations in patients, and how clinical observations can be taken back to the lab for mechanistic studies in mice.

Our People
The lab is led by Adam Bauer, PhD, and comprised of a diverse and interdisciplinary team with backgrounds in physics, engineering, computer science, biology and the neurosciences.
Projects
Our research includes wide field optical imaging of brain activity, optogenetic mapping of brain circuitry, manipulating functional recovery after stroke, cellular contributions to neurovascular coupling and light-based motor mapping using deep learning.

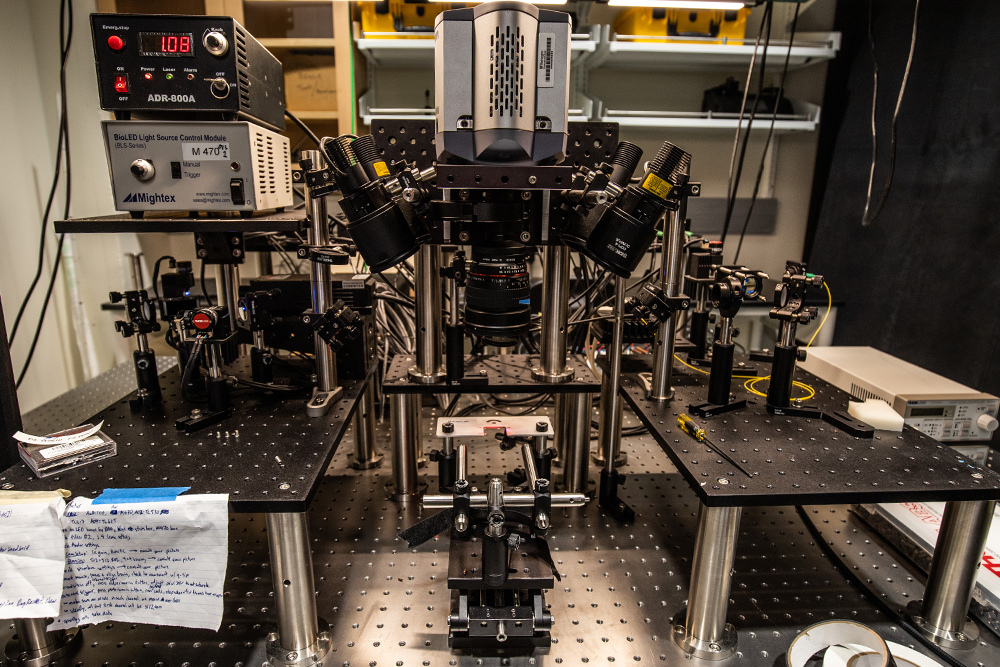
Lab Resources
By designing and building all of our imaging systems and software analysis algorithms, we’re able to tailor an imaging modality to the biological question being asked.
Lab Life
The Bauer Lab enjoys getting together for a variety of social events. We also have a mild obsession with plants and there is a fish tank (not used for research).