The chemical composition of lunar soil
As a lunar geochemist I have been approached many times by people who believe that they have a sample from the Moon. Common stories are (something like) “This dust was given to my late grandfather by astronaut Buzz Lightyear” or “This rock that I found in my petunia pot looks just like lunar meteorite QUE 94281 on your website.” Lately, people have been sending me reports that they have obtained of chemical analyses from labs or one of those hand-held x-ray “guns.” So, here is what you need to know in order to interpret those reports.
Major Elements – In lunar rocks and soils 99% of the mass
consists of the following 7 chemical elements
Oxygen (41-45%) | Silicon (Si) | Aluminum (Al) | Calcium (Ca)
Iron (Fe) | Magnesium (Mg) | Titanium (Ti)
Minor Elements – Nearly all of the remaining 1% consists to these 4 chemical elements
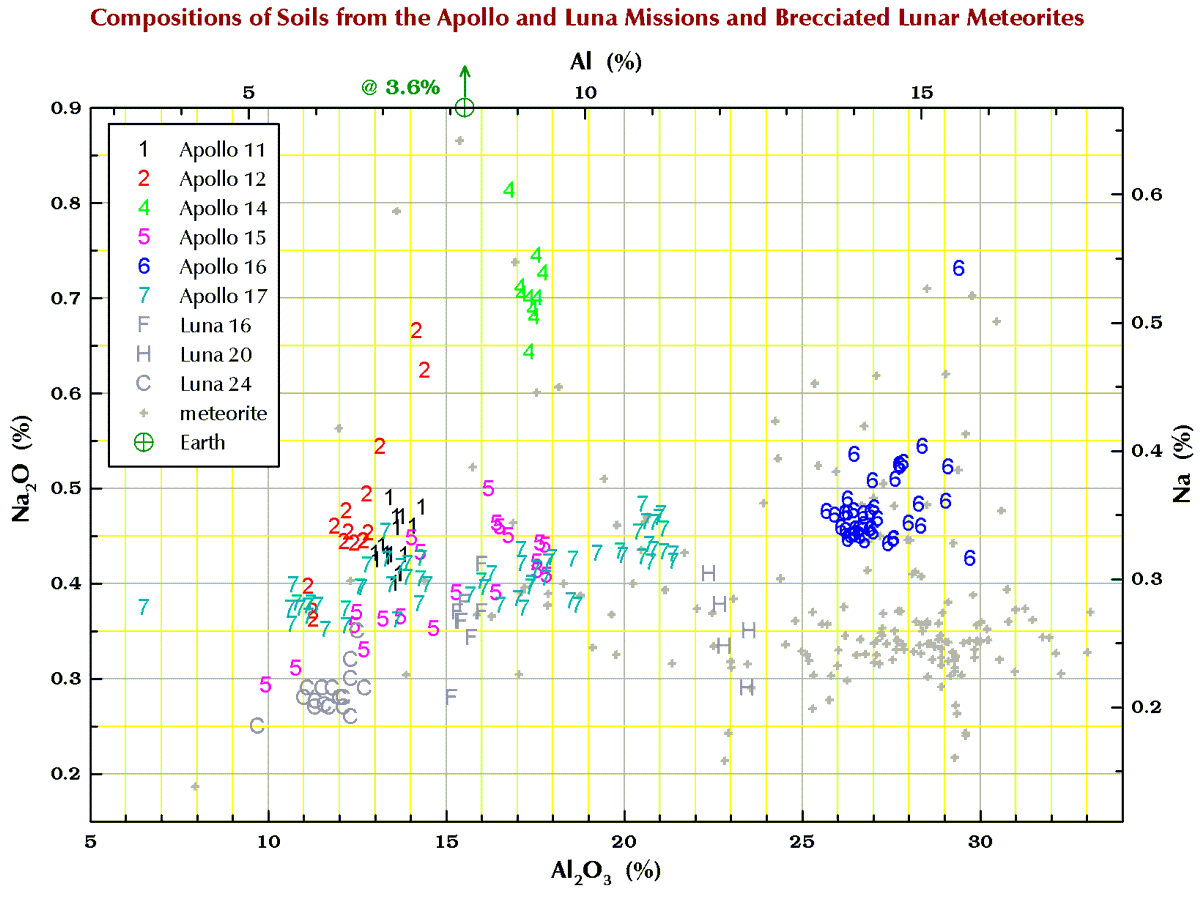
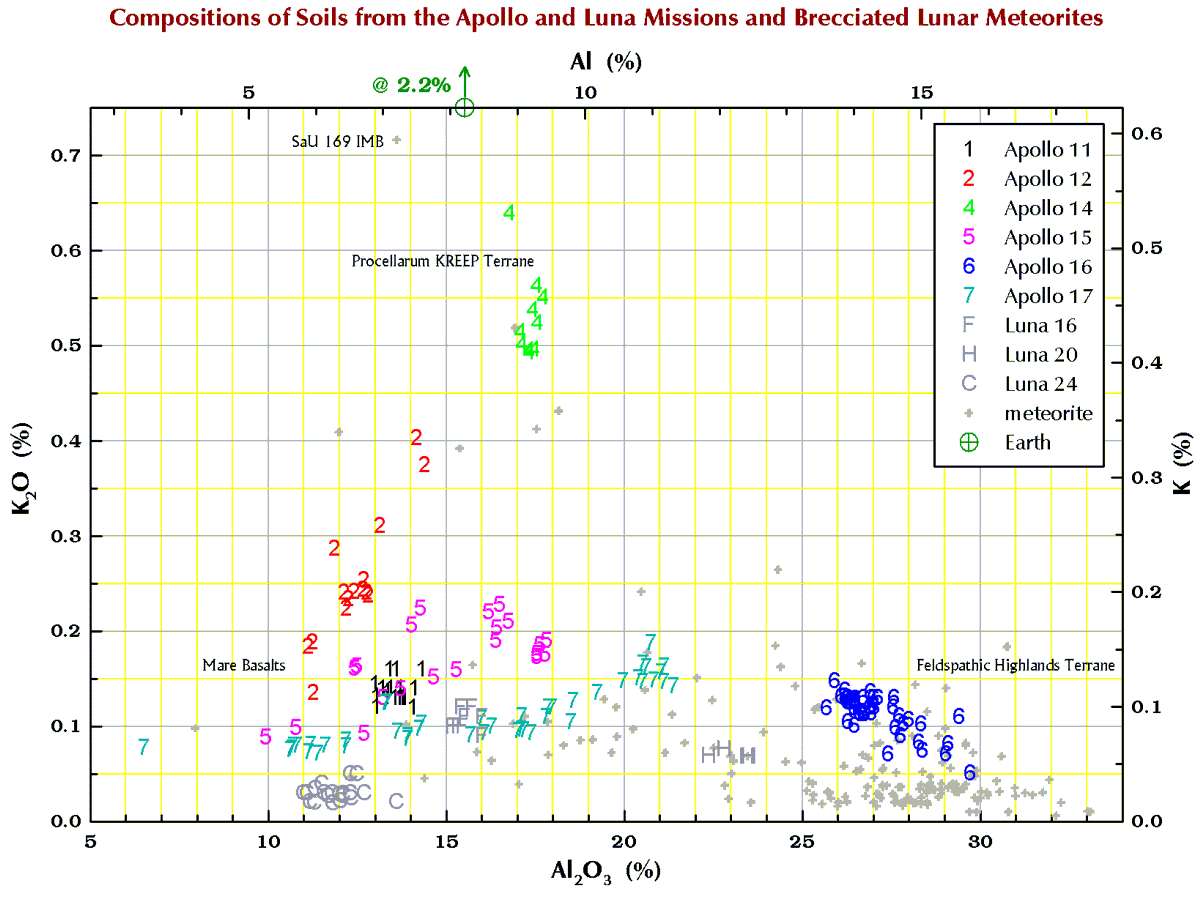
Below are charts I have made from data from dozens of literature sources and my own lab for what we geochemists call the “major elements” and “minor elements” in samples from the 6 Apollo mission and 3 Russian Luna missions that brought samples back from the Moon. To make it simple, I have stuck to just soil (regolith) samples. I have also included data for those lunar meteorites that are breccias because many to most of these rocks are composed of lithified soil. The lunar meteorites come from all over the Moon whereas the Apollo and Luna mission all come a small area of the nearside.
In rocks of the Earth and Moon, oxygen is the most abundant chemical element, 41-45% on the Moon. Practically nobody actually measures the concentration of oxygen in rocks anymore. We measure the “metals” like iron and aluminum.
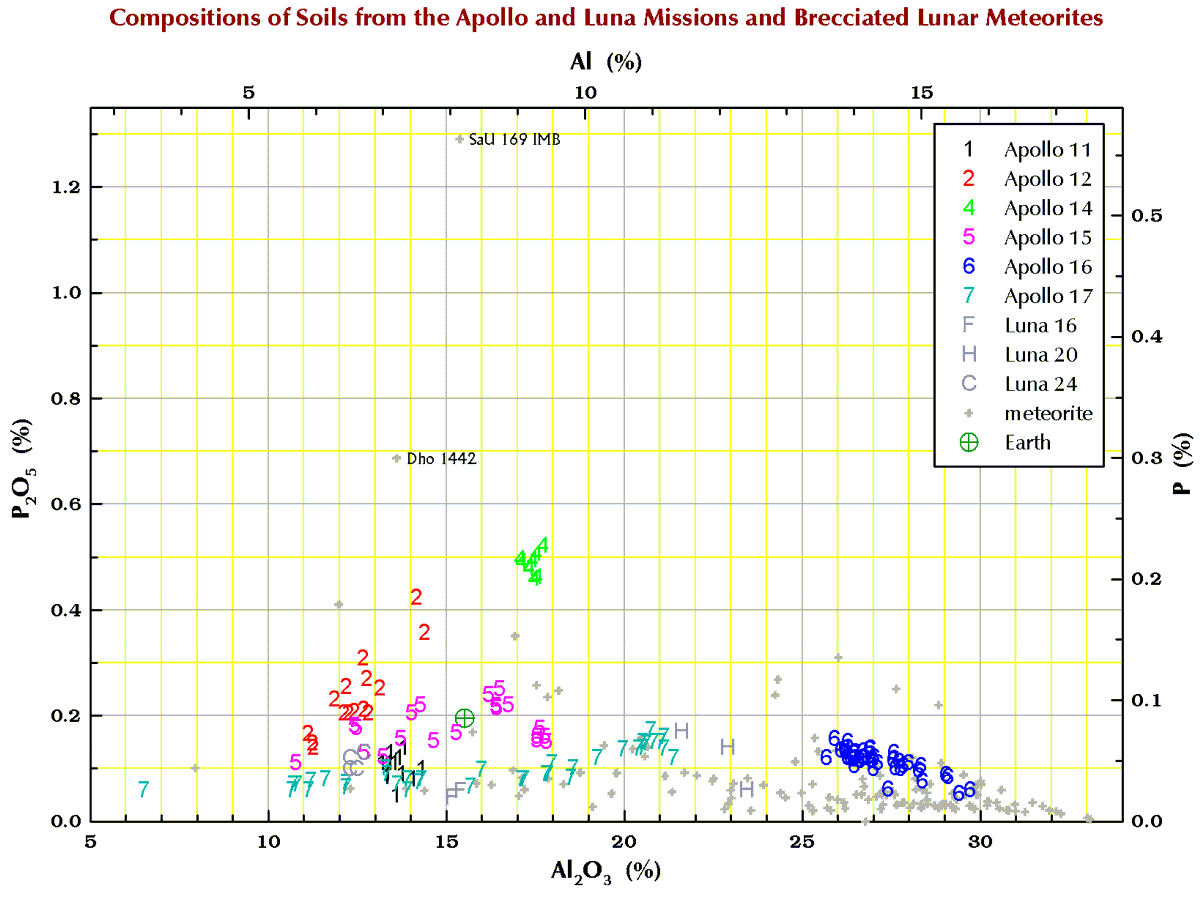
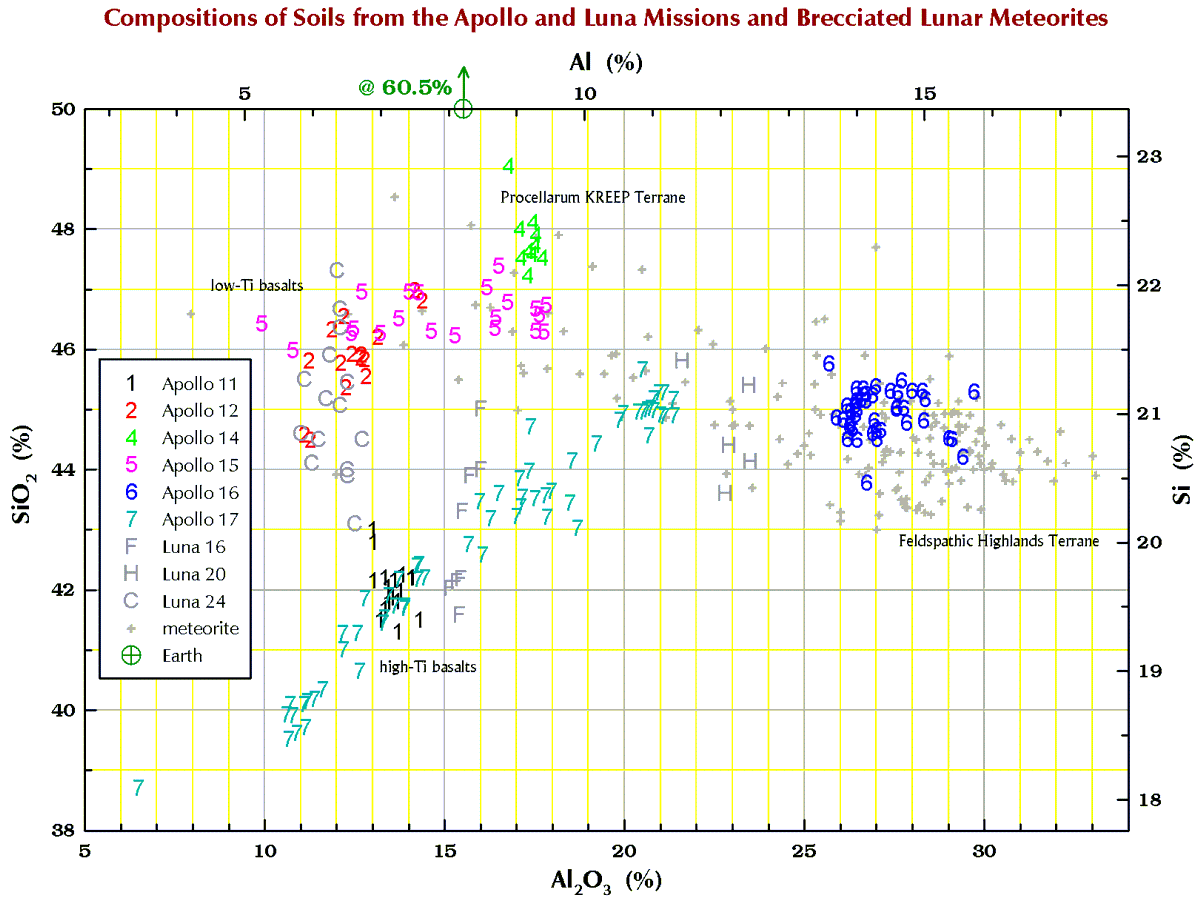
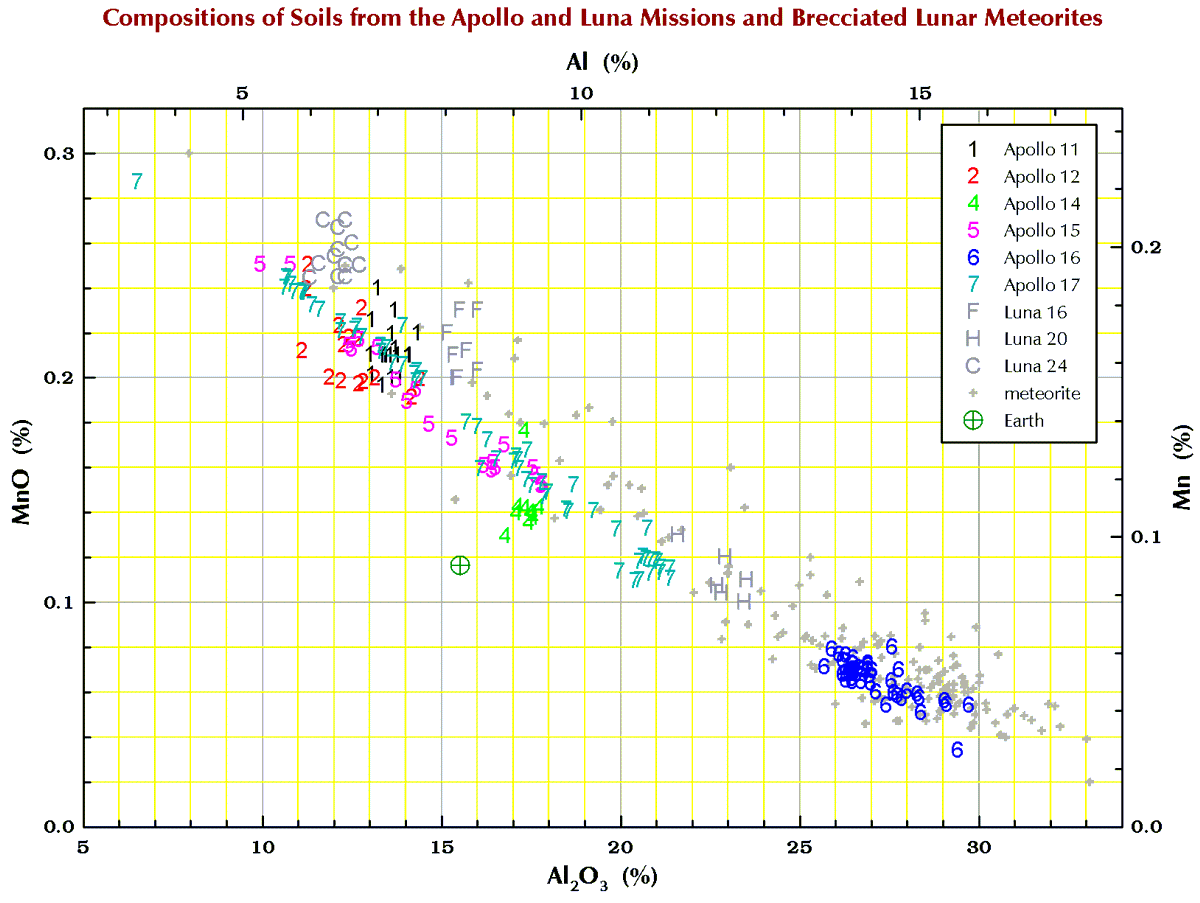
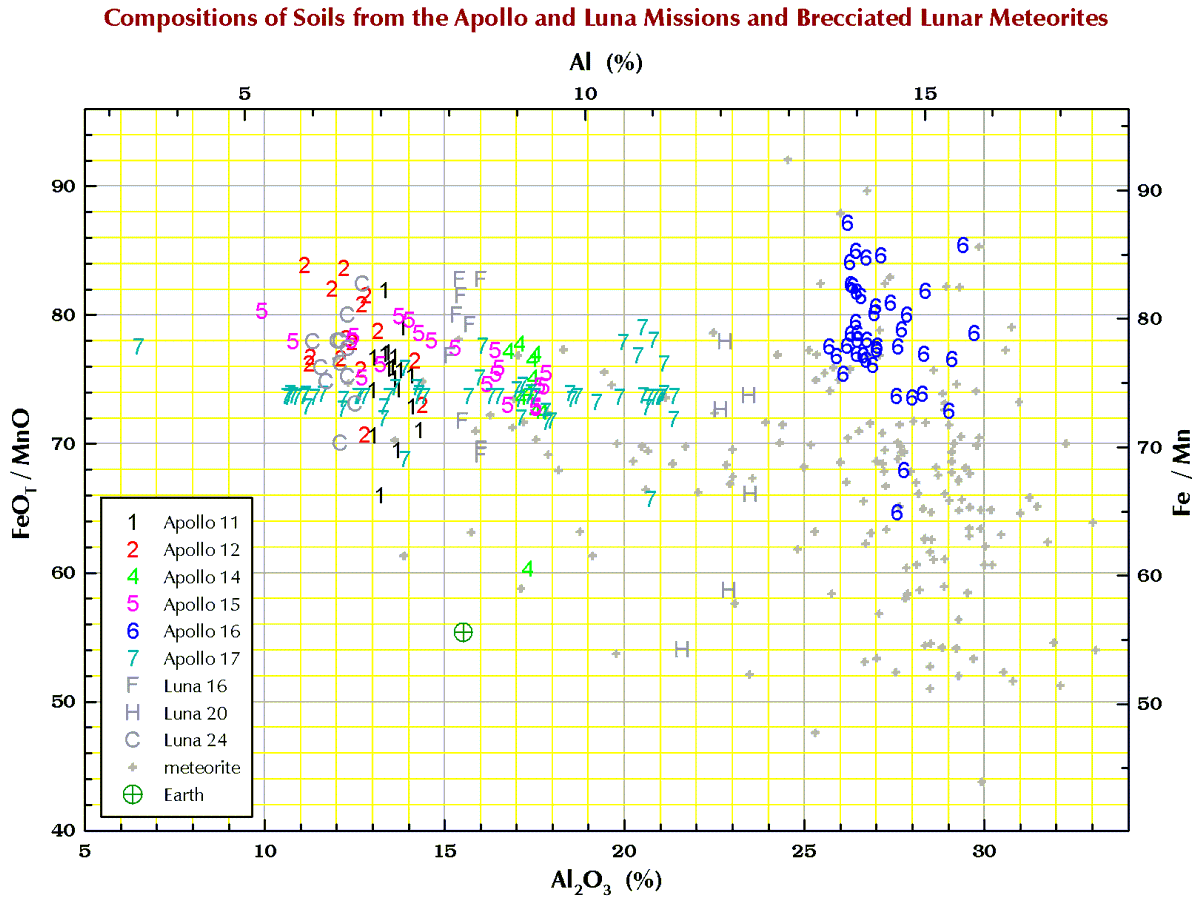
Terrestrial geochemists like to “express” the measured concentration of, say, silicon “as the oxide.” They measure the concentration of Si and state the concentration as the SiO2. So, 10.0 % Si is 21.4% SiO2. Quartz is a form SiO2, but quartz is rare on the Moon. Nearly all (>99%) lunar Si is in the silicate minerals plagioclase, pyroxene, and olivine. Likewise, there is no actual MgO (the mineral periclase) on the Moon; magnesium is carried mostly by the minerals pyroxene and olivine. We express the metal concentrations as oxide concentrations because the sum of 10 major and minor metal oxides above should be 100±1%. If not, something wrong (!) as there are no (= insignificant amounts of) carbonates, sulfates, or hydrous (water-bearing) minerals on the Moon. Lunar meteorites, however, often to contain carbonates, sulfates, or hydrous minerals as a result of weathering on Earth after they land.
So, for geochemists, the bottom and left axes of the plots below are in weight-percent oxide. For scrap-yard dealers and jewelers who might have an x-ray gun set to the “metal” setting, use the top and right axes.
All the plots have aluminum concentrations on the horizontal axis. I do it that way because Al varies over a large range in lunar samples. (To confuse you, elsewhere here I have put FeO+MgO on the horizontal axis, but that is OK because there is a strong anticorrelation between Al2O3 and FeO+MgO in lunar samples.)
Finally, in the plots below, each point for Apollo 11, and the 3 Luna missions represents a chemical analysis. For example, nearly all the Apollo 11 points represent soil sample 10084, which is probably the most well characterized geologic sample ever analyzed. For Apollos 12, 14, 15, 16, and 17, each point represents a numbered soil sample (“surface” and “trench” soils, no cores), e.g., samples 12032, 14163, 15071, 65701, and 76501 (mean of all available analyses for each). The large spread for some of these missions reflect the compositional variation among the various locations at which samples were collected at the site. For the lunar meteorites, each point represents a named stone, e.g., MacAlpine Hills 88105 or Northwest Africa 8046 and its pairs. For reference, each plot also includes an “Earth” point, which is an average of 4 different estimates I found in the literature for the mean composition of upper continental crust of the Earth.
Silicon (Si)
Iron (Fe)

Manganese (Mn)
Iron/Manganese (Fe/Mn)
Magnesium (Mg)
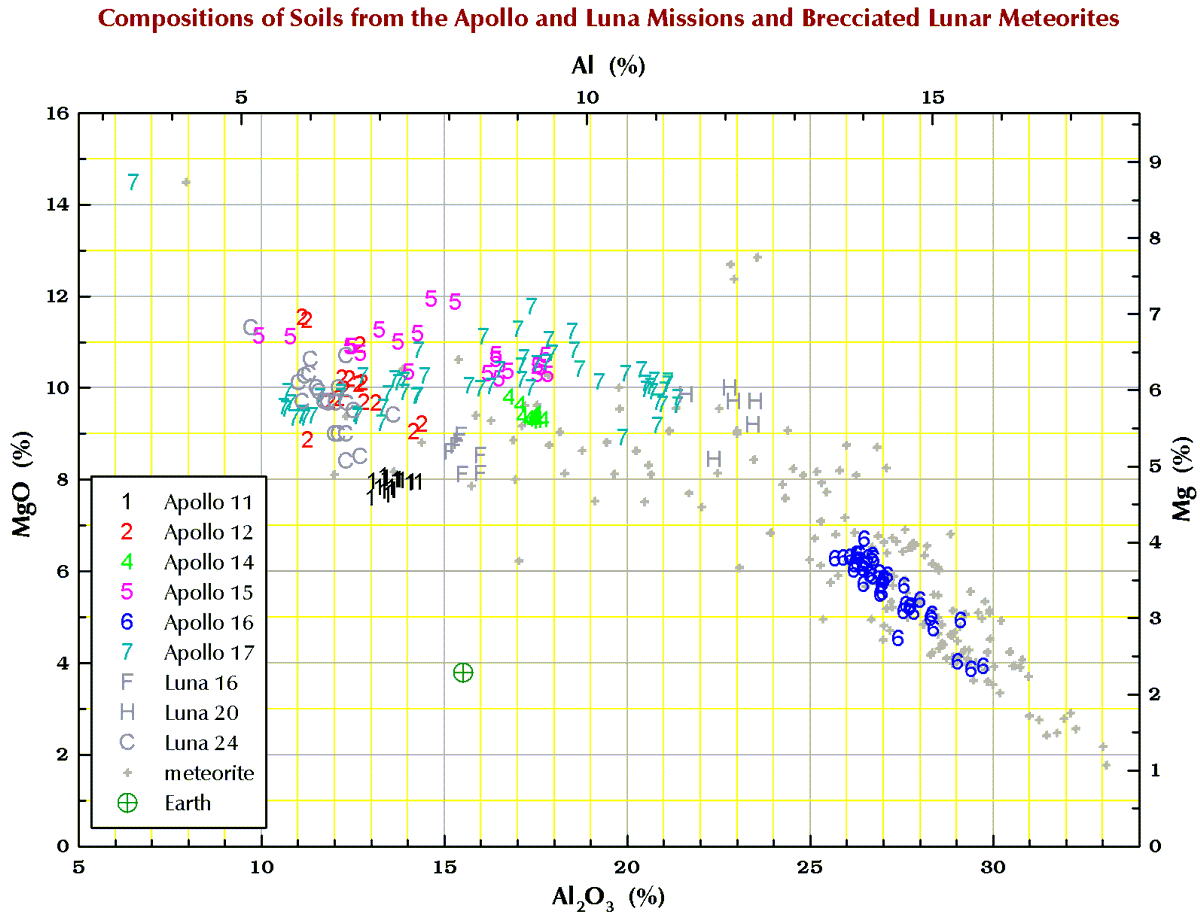
Calcium (Ca)

Calcium Aluminum (Ca/Al)

Titanium (Ti)

Chromium (Cr)

Sodium (Na)
Potassium (K)
Phosphorus (P)